Leo Wan’s cultured mouse cells didn’t look right. Or rather, they looked left, Wan noticed, as he peered at them through a microscope around 2009. Huddled in a single layer along a narrow glass slide, the cells—in this case myoblasts, progenitors of muscle cells—were the latest of hundreds that Wan had plated using a technique called micropatterning, which lets researchers adhere cells to a surface in highly regular patterns, and which Wan had been perfecting as part of his postdoctoral research at Columbia University. He’d originally assumed that these long, slender cells would align themselves with the length of the slide. But instead, the cells seemed to be pulling slightly to the left, Wan tells The Scientist.
“At the beginning, I [thought] that it’s just random,” Wan recalls. “But after it appeared so many times,” almost always in the same direction, he and his supervisor, bioengineer Gordana Vunjak-Novakovic, agreed that Wan should pivot from his current project to look into what was going on.
After measuring the tilt of the myoblasts growing in rectangular strips and also in rings, where the patterns could continue all the way around in a circle, Wan began to suspect he was observing an intrinsic bias of the cells to align one way instead of the other: although they occasionally pulled right—what Wan describes as clockwise—or didn’t show an obvious bias, about 80 percent of the time they pulled left. He became surer still when he discovered that the direction of this bias seemed to vary by cell type. “Some of them are clockwise and some are counterclockwise,” he says. Like the mouse myoblasts, human muscle cells had a counterclockwise bias, the team reported in a 2011 PNAS paper, while many other cells they looked at, including skin, heart, and bone cells, tended to be clockwise. Cancerous skin cells were an exception; their bias was in the opposite direction from their noncancerous counterparts. For Wan, now at Rensselaer Polytechnic Institute in New York, the work was an introduction to an odd quirk of animal biology: cell chirality, a little-understood phenomenon that a handful of researchers have been documenting over the last several decades in all sorts of cells.
Broadly speaking, chirality is a property of an object that is asymmetric in every plane, such that said object can’t be superimposed on its mirror image no matter how it’s rotated. The idea is intrinsically linked with the concept that some asymmetric objects can be classified as right- or left-handed based on their specific structures: a clockwise spiral is said to be right-handed, for example. Although it can be a tricky concept to envision and articulate, chirality is a familiar feature of biology, from the scale of tiny molecules all the way up to whole organisms. Many biopolymers such as DNA form helices, which are inherently chiral structures, while compounds such as amino acids form complex 3D shapes that also can have left and right forms. Researchers now know that a molecule’s handedness is critical in determining its function, and that living organisms are picky about which forms they use: while right and left versions of many molecules exist, almost all organisms exclusively synthesize and metabolize left-handed amino acids, but right-handed sugars, for example.
Chirality is a property of an object that can’t be superimposed on its mirror image no matter how it’s rotated.
Chirality is evident at the macroscale too, in the body plans of animals—even ones that outwardly appear to be symmetrical. Humans, in addition to being obviously asymmetric from head to toe and from back to front, are also asymmetric along the third axis—that is, from left to right. Major organs such as the stomach and the liver are always on the left and the right, respectively (except in very rare conditions, such as situs inversus totalis, in which a healthy person has all the organs in mirror position; see “Errors in Asymmetry” below). Chirality can be seen within individual organs, too: the heart, for example, is structurally asymmetric from left to right as well as across the other two axes.
How these body-level asymmetries arise presents enduring mysteries in animal development, notes Tufts University’s Michael Levin, an editor on Wan’s 2011 paper—and the left-right axis in particular is challenging to understand and study. While up-down and backward-forward axes have real-world meaning, such as the direction of gravity or the direction in which animals or polarized migrating cells move, there’s no clear equivalent for the third, left-right axis. “If you try to explain to an alien … and you say ‘OK, my left hand is—’ Well, what does ‘left’ mean?” Levin says. “That’s a really hard problem.”

For Wan and several other researchers, chirality at the level of individual cells could be a crucial piece in this puzzle. The phenomenon has by now been widely described. It’s known that the ciliate Paramecium tends to spiral to the left as it swims, for example; neutrophil-like cells—a cell line developed to study the migration of these immune cells—also show a leftward preference in their movement. “I think there’s a consensus regarding cells [having] a chiral bias,” says Wan, who, alongside Levin and others, has argued that this phenomenon offers an overlooked mechanistic link between molecular and organ- or organism-level asymmetries.
“To me, there’s a really smooth continuum between asking about lateralized behavior in large organisms, like, we’re right-handed or whatever . . . [and asking about] individual cell behavior that’s lateralized,” says Levin, who has been formulating hypotheses about cell chirality and body asymmetry, often at odds with the scientific consensus in developmental biology, since the 1990s. “I think it’s exactly the same thing, [just at] a different scale.”
How cell chirality is established
Not long after Wan began investigating his left-leaning cells, on the other side of the world, postdoc Yee Han Tee had begun working with another sort of micropatterning technique to study how cells form an internal structure called the actin cytoskeleton, an important mediator of cell growth, movement, and intracellular transport. Working in the lab of Alexander Bershadsky at the National University of Singapore’s Mechanobiology Institute, Tee had plated individual fibroblasts onto tiny adhesive islands that forced the typically elongated cells to adopt a circular shape, and was recording the cells through a microscope to examine how the cytoskeleton formed inside each cell over the next few hours. “One day,” Bershadsky recalls, “she came to me and said that these cells behave very interestingly.” Specifically, their insides were spinning.
Using fluorescent labeling to track the movement of individual actin fibers, the pair found that two groups of fibers seemed to be establishing a counterclockwise swirling motion within cells, Bershadsky says. So-called radial fibers grow in from the cell’s edge toward the cell’s center, forming a pattern like the spokes of a bicycle wheel, while transverse fibers that connect the radial fibers at several points move toward the center along with them, creating a pattern of concentric circles. These fibers started out in a regular, radially symmetrical pattern. But a little more than three hours after the cells were plated, the spokes began to tilt, causing the whole structure to start swirling around the cell center. Finally, around the 11-hour mark, the fibers stopped swirling and stretched out more or less in parallel across the diameter of the cell.
TWISTS AND TURNSIndividual cells are chiral: in addition to showing up-down and front-back asymmetries, they are also asymmetric along the left-right axis. One of the possible ways this chirality could get established inside cells is through the spontaneous self-organization of the actin filaments making up the cell cytoskeleton. In one study a few years ago, Yee Han Tee and colleagues plated fibroblasts individually onto circular adhesive islands that forced each cell to maintain a circular shape, and imaged the movements of two sets of actin fibers in each cell: radial fibers, which stretch inward from focal adhesions on the cell membrane, and transverse fibers, which are arranged perpendicularly to the radial fibers and seem to physically interact with them. In the first few hours of cytoskeleton development, these fibers formed a radially symmetrical pattern (left), but after about three hours, the radial fibers began to tilt, dragging the transverse fibers sideways and causing a swirling pattern to emerge (middle). Then, at around the 11-hour mark, the swirling broke into a linear pattern of fibers stretching across the cell (right).  illustration by © scott leighton; DATA FROM NAT CELL BIOL, 17:445–57, 2015. |
While the idea of cytoskeleton chirality wasn’t new, the experiments, published in 2015 alongside a computational model replicating the swirling, provided one of the first visualizations of this chirality arising spontaneously from molecules inside the cell. Digging further into that mechanism, Bershadsky, Tee, and colleagues used small-molecule drugs to inhibit actin-associated proteins, and found that this caused the cell cytoskeleton to lose its chirality or even swirl in the opposite direction. Other groups have pursued the idea, too. Researchers in Japan studying zebra-fish pigment cells called melanophores reported several years ago that the cells’ tendency to rotate counterclockwise in culture could be abolished by treatment with inhibitors of actin assembly.
As actin itself is a chiral molecule—it forms a right-handed helix—Bershadsky, Levin, and others have speculated that its molecular structure could be central to the establishment of cellular asymmetry. “Our [view] is that . . . [the] actin filament, and its helical asymmetry, is their chirality factor,” Bershadsky says. While it’s still unclear exactly how the mechanism might work, it could be that this structure influences how actin filaments respond to mechanical forces and interaction with other intracellular proteins, he adds.
Actin fibers aren’t the only component of the cytoskeleton, though, and other labs have investigated a role for micro-tubules—stiffer, thicker fibers that play a bigger role than actin in certain intracellular processes such as vesicle transport. In one early study of chirality in neutrophil-like cells, researchers used pharmacological treatments to disrupt microtubule assembly, reporting that while cells could still migrate, they no longer showed a leftward bias in their movement. A few years later, Levin and colleagues similarly found that asymmetry was abolished in neutrophil-like cells when they knocked out tubulin, the protein that makes up microtubules.
Researchers disagree about the importance of these different elements in establishing cell chirality. Bershadsky notes that microtubules swirled along with the actin fibers in their experiments, but that disrupting microtubule function didn’t interrupt the swirling or affect its direction. The group studying melanophores reported that cells actually rotated more when treated with microtubule inhibitors. Levin, who has also studied actin-associated proteins, says he thinks that both actin and microtubules are probably involved. “The evidence is pretty good for both of them; I don’t think there’s any need to pick one.”
To get a better sense of how involved these various elements are in driving chirality at the macroscale in living organisms, some groups are trying to observe cell chirality in setups designed to more closely mimic natural settings. For example, Wan and colleagues developed a 3D micropatterning technique to better replicate the embryonic environment and used the setup to measure rotational behavior in spheroids of epithelial cells. (Wan is listed as an inventor on patents related to micropatterning techniques in cell chirality research.) The team found that the majority rotated counterclockwise, but that pharmacologically blocking actin assembly resulted in a switch, with most of those spheroids rotating clockwise.
The same group is also trying to develop methods to identify cell chirality from visual features, such as the distribution of intracellular organelles, to pave the way for in vivo observations. One approach, they suggest in a recent paper, could be to note the position of the cell’s center of mass with relation to an imaginary line drawn between the nucleus, which tends to be at the rear of a moving cell, and the centrosome, which is usually nearer the front. Endothelial cells, previously found to have a right, or clockwise, bias, tend to have the center of mass to the right of this front-back axis, the researchers report in the study, suggesting the approach could provide a rough way of determining a cell’s chirality.
Wan and others are also studying what happens when many chiral cells get together—in the kinds of groups that form swarms of migrating cells in an embryo, for example, or swirl together to form organs. It’s these kinds of studies that could help scientists resolve the more controversial issue of just how important cell chirality is in animal development.
ERRORS IN ASYMMETRYAlthough humans and other bilateral animals outwardly seem to have more or less symmetrical right and left sides, there are a number of significant physiological and genetic asymmetries that arise during normal development. Errors in the development of these patterns can lead to various unusual conditions in left-right patterning, two of which are illustrated below. |
 modified from © istock.com, reklamlar Situs inversus totalisIn normal human development, many major organs end up on one side of the body’s midline—the liver on the right, the heart slightly to the left, and so on. But in around 1 person in 10,000, this arrangement is flipped, with organs usually on the right developing on the left, and vice versa. The condition of having all organs on the opposite side from normal, known as situs inversus totalis, isn’t usually harmful, since the relationship between organs is the same. However, related conditions where only some organs’ positions are flipped, such as levocardia (where everything but the heart is misplaced), dextrocardia (where only the heart is on the wrong side), or situs ambiguus (where there’s a mix of flipped and not-flipped organs), are more commonly associated with heart defects and other medical problems caused by organs failing to interact properly with one another. Analogous conditions are seen elsewhere in the animal kingdom. For example, in the last few years, researchers at Nottingham University have studied the determination of spiral direction in the shells of the great pond snail (Lymnaea stagnalis). While most snails in this species have right-spiraling shells, the team recently found some with left-handed spirals, and has been investigating the phenomenon’s genetic basis. |
 modified from © istock.com, o-che GynandromorphismOriginally observed in invertebrates, gynandromorphs—organisms that contain both male and female characteristics—have now been documented in numerous animal species, including rodents and many species of birds. One version of the phenomenon, known as bilateral gynandromorphy, occurs when the split in characteristics such as coloring falls directly along an animal’s midline, creating a stark difference in the appearance of left and right sides. The differences seem to start arising from differential gene expression very early on in animal development, and the phenomenon has been taken by some scientists as evidence that a distinction between left and right sides occurs when developing embryos are just a handful of cells. |
Cell chirality’s role in asymmetry at larger scales
By the early 21st century, an important piece of the puzzle of how left-right asymmetry gets established in the developing vertebrate embryo had apparently been solved: tiny beating hairs called cilia that assemble on the edges of some cells create a leftward flow of fluid across the ventral side of the embryo after the anterior-posterior and dorsal-ventral axes are established. This flow triggers asymmetric gene expression depending on a cell’s position along that left-right axis, and eventually divides the body into left and right sides. Knockout experiments showed that eliminating proteins required for cilia assembly—such as kinesins, which transport the building blocks for cilia along microtubules—disrupted or reversed the flow of fluid in mice embryos and produced animals with incorrectly located organs. And genetic screens of mice with laterality defects turned up mutations in dozens of cilia-associated genes. Cilia, it seemed, were the symmetry breakers for the elusive third axis.
While that view has remained dominant among researchers studying animal development, Levin points out that most studies ignore the fact that knocking out cilia-associated proteins could also have effects on the cytoskeleton and intracellular processes. Moreover, he notes, left-right asymmetry is also established in animals such as chicks, pigs, and roundworms, whose embryos lack ciliated cells to establish fluid flow. And even in animals with cilia, such as frogs, he adds, it’s possible to detect asymmetric distributions of RNA and other key developmental molecules long before the stage at which the hairs assemble and start beating. Knocking out tubulin disrupts laterality development across animal species and even in plants, suggesting instead there may be a universal mechanism of establishing asymmetry that is dependent on tubulin, but not on cilia.
As actin itself is a chiral molecule, some researchers speculate that its molecular structure could be central to the establishment of cellular asymmetry.
“It’s completely implausible to say that cilia are the initiator of asymmetry,” Levin says. “I think the most charitable thing you can say is that it’s fairly reasonable to think that cilia are involved in some fashion in the middle of the pathway,” perhaps amplifying differences in left-right asymmetry already established by intracellular mechanisms.
The details of potential non–cilia-based mechanisms in establishing tissue- and body-level asymmetry are far from resolved. Researchers who spoke to The Scientist distinguished between two aspects of the problem that scientists studying cell chirality need to address. One is how cells could provide tissue-level directional information in the first place—that is, which way is left and which way is right? The second, more challenging question is how a cell could encode positional information—that is, where’s the middle of the embryo, and which side am I on?
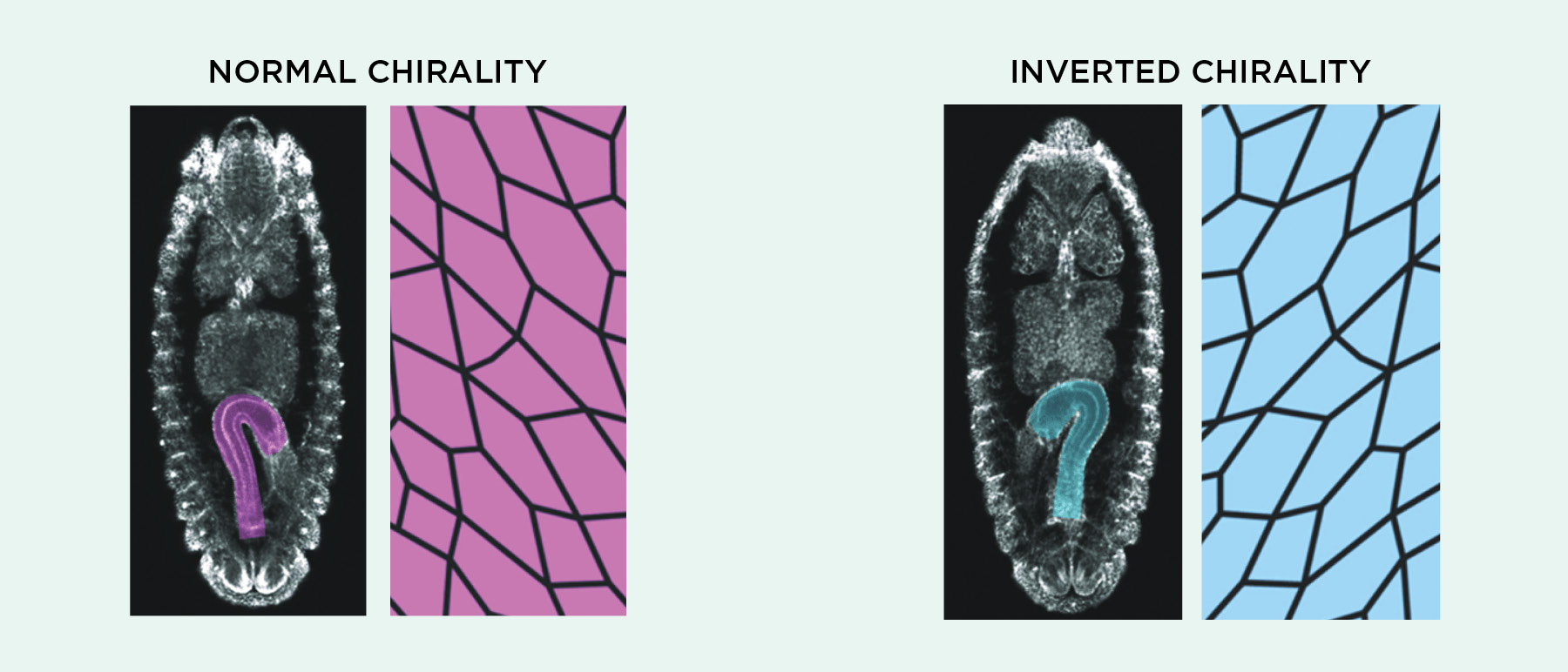
In answer to the first question, many groups have shown that cell chirality consistently manifests itself at the group level in vitro as cells align themselves with one another. Like Wan’s left-leaning mouse cell layers, Tee’s fibroblasts showed collective patterns in alignment and migration that could be eliminated or reversed by treatments such as actin-disrupting drugs, according to a preprint posted last April. Several researchers have shown that this kind of collective action can have consequences at the level of whole tissues and organs. Kenji Matsuno, a fruit fly researcher at Osaka University in Japan, has been studying asymmetry in the fly embryonic hindgut, which undergoes a 90-degree left rotation during development. The epithelial cells making up this gut tube themselves are asymmetrically shaped, Matsuno tells The Scientist, and his group has shown that disrupting actin-associated proteins not only makes these cells flip handedness, but also can reverse the direction of hindgut rotation. The team argues in a recent paper that cell-level chirality is both necessary and sufficient to drive the phenomenon.
Wan, meanwhile, has been working on avian heart development. The heart is one of the first organs to break symmetry in the embryo, a process that begins with a tube of cells that typically forms a rightward loop. Wan’s group has reported that cells isolated from chick embryo hearts show an intrinsic right-handed bias, and that this bias could be flipped in culture using various drugs known to disrupt actin cytoskeleton chirality and other intracellular structures. Trying the treatments on chick embryos in vitro caused many of them to developed left-looping hearts. “This gives us some kind of evidence that cell chirality highly possibly plays a role there,” Wan says. He adds that the one time his team chanced upon a chick embryo that naturally had a left-looping heart, “we found that those cells are counterclockwise, just as if they were treated with small-molecule drugs.” He and others are now expanding the work to study potential implications of cell chirality for human health and disease, with regard to heart development and to other phenomena, such as the permeability of endothelial cell barriers and competition between cancerous and normal cells.
While such mechanisms offer explanations for how asymmetric organs develop, it’s less clear if they drive the higher-level asymmetry evident in animal body plans. Researchers agree that at some point during development—it varies considerably among species—a kind of molecular barrier forms along the embryo midline to block diffusion of growth factors from one side to the other, aiding the accumulation of asymmetric gene expression. But Levin points out that cases of lateral patterning abnormalities such as gynandromorphy, where one half of an animal is genetically male and the other is female, imply that there’s a fundamental left-right split that forms much earlier on.
“These genetic disorders wouldn’t be down the middle if those genetic issues showed up later in development,” says Levin, who has published models to describe how cell chirality, and specifically direction-biased transport of particular intracellular proteins along the cytoskeleton, could help give rise to these phenomena by establishing voltage or pH gradients across the embryo. For now, he argues, the true mechanisms remain unresolved. “It’s a total puzzle.”
Puzzles remaining in cell chirality research
Whatever cell chirality’s role in animal development, researchers who spoke to The Scientist acknowledged that there are some fundamental questions that need addressing. For a start, it’s not clear why some cells show clockwise biases while others show counterclockwise ones, or how those differences get established. Wan says his colleagues have pointed out that muscle cells tend to contain more actin than other cells, perhaps helping explain why mammalian myoblasts pull left when many other cells pull right or don’t show much directional bias at all. He adds that endothelial and epithelial cells typically have opposing chiralities, a phenomenon he says he’s interested in studying further.
Some researchers also view the fact that cells and cell populations aren’t 100 percent consistent in the way they lean as a limitation of the field, as it means that studies rely on statistical approaches to identify chiral bias. After all, in Wan’s 2011 paper, only about 80 percent of the myoblast populations showed a counterclockwise bias—far lower than the percentage of amino acids in the human body that are left-handed, or than the percentage of embryonic vertebrate hearts that form a right-handed loop. The same is true in studies of individual cells: while most show chirality in one direction, there are always a few that lean the other way.
Cell chirality may not be a zero/one switch. I now believe it’s quite complex.
—Kenji Matsuno, Osaka University
Molecular engineer Jiandong Ding of Fudan University in China and colleagues have argued in several papers that this low consistency is a common theme in cell chirality research, and have called for caution in interpreting the results. It’s not clear whether the inconsistency arises from experimental artifacts or other confounding factors, or whether it reflects true variation among cells. But Wan argues that cell chirality is important, noting the repeatability of studies that change the chirality of cells and whole organs using cytoskeleton-disrupting drugs. Even when chirality isn’t uniform in a cell population, he adds, it may be sufficient to drive tissue-level behavior, particularly if it’s just one of many mechanisms involved in establishing and amplifying asymmetry during development. Matsuno adds that some researchers are coming to view chirality as less of a binary phenomenon, and more of a spectrum that includes both strong and weak biases in either direction. “Cell chirality may not be a zero/one switch,” he says. “I now believe it’s quite complex.”
Solving these puzzles will be part of the research agenda for the field going forward, says Bershadsky, who’s working with Wan to organize sessions on cell chirality and symmetry breaking at the World Congress of Biomechanics this July. The area is “still fresh—and this is why we like it,” Bershadsky says. He adds that after several years studying chirality across the scales of biology, he now finds himself more surprised that animal body plans are as close to being right-left symmetrical as they are. “The fact that the majority of animals have this bilateral . . . symmetry is something that’s not so easy to understand,” he says. “The beauty is that nature somehow formalized and codified these deviations” from this symmetry, he says. “They are not random. They are very well inherited and regulated.”

