 Injection of an adenoviral vector encoding pancreatic transcription factors induced insulin expression (red) in mouse pancreatic ducts. Ductal tissue is shown in green and nuclei in blue. YUHAN WANGOne strategy to treat type 1 diabetes, where the immune system destroys insulin-producing β cells, is to convert other cells into β-like cells that then take over insulin production. In a study published today (May 2) in Molecular Therapy, researchers reprogrammed pancreatic duct cells in vivo to successfully treat both genetically and chemically induced diabetes in mice.
Injection of an adenoviral vector encoding pancreatic transcription factors induced insulin expression (red) in mouse pancreatic ducts. Ductal tissue is shown in green and nuclei in blue. YUHAN WANGOne strategy to treat type 1 diabetes, where the immune system destroys insulin-producing β cells, is to convert other cells into β-like cells that then take over insulin production. In a study published today (May 2) in Molecular Therapy, researchers reprogrammed pancreatic duct cells in vivo to successfully treat both genetically and chemically induced diabetes in mice.
“The work is part of a long line of studies attempting to genetically engineer insulin-producing cells in various tissues,” says Jake Kushner, a pediatric endocrinologist at Baylor College of Medicine in Houston who did not participate in the study. “It’s an exciting advance because it refines our understanding of what the capacity is of the various different components of either the...
See “Gene Therapy Temporarily Reverses Type 1 Diabetes in Mice”
Previous work had shown that both liver and pancreas cells could be converted into insulin-producing cells. Thus, Yuhan Wang, then a graduate student in Markus Grompe’s lab at Oregon Health & Science University, and colleagues set out to determine which of these cell types would make the best β-like cells.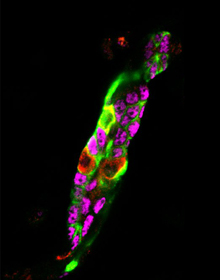 Injection of an adenoviral vector encoding pancreatic transcription factors induced insulin expression (red) in mouse pancreatic ducts. Lineage traced pancreatic ducts were identified by GFP (green). Insulin-positive pancreatic ducts were characterized by their loss of pancreatic ductal transcription factor expression, Sox9 (magenta).YUHAN WANG
Injection of an adenoviral vector encoding pancreatic transcription factors induced insulin expression (red) in mouse pancreatic ducts. Lineage traced pancreatic ducts were identified by GFP (green). Insulin-positive pancreatic ducts were characterized by their loss of pancreatic ductal transcription factor expression, Sox9 (magenta).YUHAN WANG
In the latest study, the researchers intravenously injected diabetic mice with a virus loaded with the genes for Pdx1, Neurog3, and Mafa—transcription factors that had already been shown to reprogram cells to a β-like state. The team first targeted the mice’s liver cells, called hepatocytes, and found that after the injection, the hepatocytes that were altered by the transcription factors initially made insulin and could reduce levels of high blood sugar in the mice. But the cells did not respond appropriately to low glucose levels by turning off insulin production, and their ability to make insulin didn’t last, so the mice ended up with high blood sugar again. The hepatocytes also did not express characteristic β-like genes, suggesting that they were not truly reprogrammed.
Viral injections into the duct systems of the liver and pancreas also induced insulin production in both types of duct cells. The team observed that the insulin-positive duct cells of the pancreas turned on greater numbers of characteristic β-like genes compared with the insulin-positive liver duct cells. The insulin-positive duct cells of the pancreas also turned off genes specific to the cells’ identity before transformation, while the insulin-positive liver duct cells did not. A majority of the insulin-positive cells were originally pancreatic, not liver, duct cells, the authors also found. The researchers then showed that viral injections to the ducts eliminated diabetes symptoms—namely, high blood sugar—during an eight-week experiment in 30 percent to 40 percent of the diabetic mice. The rest of the treated mice showed only transient improvements or didn’t respond to the injections at all.
“There is now growing evidence, from many different groups, that at least some specific sub-populations of cells within the pancreatic ducts can be induced to regenerate [β cells]. This could have important therapeutic implications,” Juan Domínguez-Bendala, who studies pancreatic development at the University of Miami Miller School of Medicine and did not participate in the work, writes in an email to The Scientist. “However, the use of viruses to induce this conversion is a form of gene therapy that has a very steep path to clinical approval. No matter how careful we are with viruses, they tend to elicit immune responses and create havoc wherever they land their cargo.”
Susan Bonner-Weir, a diabetes researcher at Harvard University who was not involved in the study, points out that this type of injection in people could result in inflammation of the pancreas and that, even if new β-like cells are made, they likely will be destroyed by the immune system in patients with type 1 diabetes. “It’s really a violent destructive force,” she says.
“The fear that many of us have is that if you come up with an endless supply of β-like cells, and you don’t find a way to permanently and safely reeducate the immune system, you’re going to create fuel for the autoimmune fire,” Kushner says.
Wang, who is now a postdoc at HHMI Janelia Research Campus, acknowledges these concerns and says the group plans to explore alternative delivery methods for the transcriptions factors, evaluate safety, and investigate the role that autoimmunity could play in the approach.
“We have some solid functional data showing that these cells can be reprogrammed in vivo and reverse the diabetes in these animal models,” she says. “We hope our future work will provide us additional information to address the question [of] whether this would be a valid approach for clinical treatment.”
Y. Wang et al., “Long-term correction of diabetes in mice by in vivo reprogramming of pancreatic ducts,” Molecular Therapy, doi:10.1016/j.ymthe.2018.02.014, 2018.
Interested in reading more?





