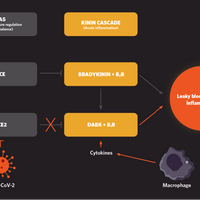
Interplay between the kinin pathway, which mediates acute inflammation; the renin-angiotensin system (RAS), which regulates blood pressure and fluid balance; and macrophages, immune cells that are activated in infection, leads to leaky blood vessels and inflammation in some cases of SARS-CoV-2 infection.
Researchers propose that the kinin cascade—in which bradykinin and des-Arg9-bradykinin (DABK) are major proteins—goes into overdrive to cause these effects during COVID-19. Ordinarily, the RAS—in which angiotensin-converting enzyme (ACE) and ACE2 are key enzymes—keeps the kinin cascade under control. ACE breaks down the protein bradykinin, preventing it from binding to its receptor B2R, while ACE2 degrades DABK and stops it from binding to B1R. When SARS-CoV-2 hooks up with ACE2 as a means of entering cells, some of the brakes are removed, according to the model, thereby permitting DABK to bind to its receptor and trigger blood vessel leakage...
Activated macrophages secrete proinflammatory cytokines such as interleukin-1 and tumor necrosis factor-alpha, which in turn induce B1R to amplify the inflammatory milieu seen in COVID-19.
Read the full story, “Is a Bradykinin Storm Brewing in COVID-19?”
Interested in reading more?