ABOVE: MODIFIED FROM © ISTOCK.COM, DR_MICROBE
On July 18, 1921, the first infant was inoculated with a live bovine strain of bacteria (Mycobacterium bovis). His mother had died from an infection with the closely related human pathogen M. tuberculosis following his birth at a Paris hospital a few hours earlier. The child’s grandmother, who would care for him, also had tuberculosis (TB). In an attempt to protect the newborn from the disease, doctors gave him an oral dose of what was later named Bacille Calmette-Guérin (BCG) vaccine, for its developers Albert Calmette and Camille Guérin. The duo had cultured M. bovis for more than a decade until it no longer caused disease in animals.
See “Bile and Potatoes, 1921”

Nowadays, BCG is given to more than 100 million babies each year, primarily in the developing world, and saves tens of thousands of lives. But it provides incomplete protection, and TB remains the number one infectious killer on the planet. It is estimated to have wiped out 1 billion people in the past 200 years, including 1.4 million in 2019 alone. This colossal burden is made even more tragic by the fact that doctors now have antibiotics to treat it. Stephen Gordon, an infection biologist at University College Dublin, tells his students that TB is “the forgotten pandemic.”
Although BCG is currently the only available TB vaccine, researchers have for decades been working to develop a better option. And after trialing dozens of approaches, with a handful of clinical failures, those in the field express hope that a second vaccine is close to its market debut. “We have a rich pipeline—lots of candidates,” says Thomas Hawn, an infectious disease scientist at the University of Washington. “That gives me optimism.”
BCG’s shortcomings
While TB is often thought of as a severe lung disease, inflicting chest pain and an often bloody cough, in children infections more commonly move beyond the lungs. This can take the form of miliary TB, when the disease affects multiple organs and is almost always fatal without antibiotic treatment, and TB meningitis, when the membranes around the brain and spinal cord become infected. One of BCG’s biggest benefits is that it prevents these forms of infection. “BCG given at birth confers consistent reliable protection against disseminated TB in children,” says Helen McShane, a vaccine researcher at the University of Oxford.
In adolescents and adults, who together account for more than 90 percent of TB cases, tuberculosis presents as the more familiar lung infection, and for some reason, BCG is less effective at preventing this form of the disease. “It confers more variable protection against lung disease, which is where the burden of TB now lies,” says McShane.
Strangely , the strength of protection conferred by the BCG vaccine to adolescents and adults varies with geography. The vaccine has good efficacy in Scandinavia and other high-latitude locales, but protection is weaker in populations closer to the equator. A favored explanation is that other species of mycobacteria found in equatorial regions can trigger the human body to generate an immune memory that recognizes and reduces replication of the BCG organism. “Exposure to nontuberculosis mycobacteria is highest in the areas of the world where BCG seems to work less well, so that’s the epidemiology evidence for the association,” says McShane.
Complicating matters is that M. tuberculosis can lie dormant in the human body for decades. Some 2 billion people are latently infected with the bug, and when an opportunity arises—such as when the immune system is suppressed, as is common in HIV patients—the bacterium activates, making that person sick and liable to transmit the airborne pathogen to others.
Strangely, the strength of protection conferred by the BCG vaccine to adolescents and adults varies with geography.
The key to understanding these dynamics is in the immune response, experts say. Having coexisted with humans for millennia, M. tuberculosis has evolved molecular tricks for evading our immune radar and even blunting responses when it is detected. When people breathe in M. tuberculosis, the microbe enters the lungs and seems to lure in immune cells. Alveolar macrophages that patrol the lungs will engulf TB and try to kill it, but “they unwittingly provide the very niche that the bacteria wants to get into,” says Gordon. Inside macrophages, M. tuberculosis is safe from antibodies.
The “waxy” capsule of M. tuberculosis helps cloak the bacteria to hide them from immune recognition, Rasmus Mortensen, the head of TB vaccine research at the Statens Serum Institut in Copenhagen, writes in an email. If the bacillus is recognized and ingested by a macrophage, once inside it is capable of “blocking phago-lysosome maturation, allowing it to persist and even replicate in the macrophage.” M. tuberculosis also interferes with antigen presentation, whereby frontline immune cells display TB antigens to T helper cells, “delaying the onset of protective T cell responses and limiting the effect of the T cells once primed.”
The body eventually walls off M. tuberculosis inside clumps of immune cells called granulomas, where the bacterium stays “like a time bomb,” says Gordon, “waiting for the right moment when your defenses are down.”
Not all cases are serious. Most people with an M. tuberculosis infection cope well; only 5–15 percent develop the disease. “T B and humans have been battling and sharpening their swords on each other for so long,” remarks Thomas Scriba, a TB immunologist at the University of Cape Town in South Africa, “that the balance between who gets to win and who gets to lose is a really fine one.”
The FrontrunnersThere are two vaccine candidates in Phase 3 trials, with eight at their heels in Phase 2. Further back, there are three vaccines in Phase 1 (not shown) as well as a handful of preclinical candidates jockeying to advance to human trials.  tHE SCIENTIST STAFF |
Live vaccinesVPM1002: A live, attenuated BCG vaccine with a pore-forming protein from another bacterium that allows the flow of antigens and mycobacterial DNA out from the phagosome into the cytosol (see graphic below) BCG Revaccination: A booster shot of BCG MTBVAC: A live, genetically weakened M. tuberculosis (first and only such vaccine to enter clinical trials) with mutations in virulence genes |
Protein subunit vaccinesM72 + ASO1: A recombinant fusion protein consisting of two M. tuberculosis antigens and an adjuvant (see graphic below) H56:IC31: A protein vaccine consisting of two early secretory proteins and a latency protein, together with an adjuvant ID93/GLA-SE: Fusion of four M. tuberculosis virulence antigens, combined with an adjuvant GamTBVac: A subunit vaccine that fuses two M. tuberculosis antigens with an adjuvant |
Whole cell vaccinesDAR-901: An inactivated preparation of the related species Mycobacterium obuense, which does not cause disease (see graphic) MIP: An inactivated vaccine that consists of M. indicus pranii, a rapidly growing mycobacterium that does not cause disease |
Vector-based vaccinesTB/Flu04L: A intranasally delivered live attenuated flu virus carrying two antigens from M. tuberculosis |
A fruitless search for signs of immune protection against TB
Researchers do not know what exactly BCG is doing immunologically to protect children, and they are still somewhat mystified as to precisely what immune response they need for a successful vaccine in adults. Like most vaccines, BCG is far better at stimulating the production of antibodies, which tackle microbes outside of cells, than at mustering a strong T cell response. “Almost every vaccine we have works by generating neutralizing antibodies, but for TB, we don’t think neutralizing antibodies are going to be enough,” says Gordon.
Vaccine-related correlates of protection (COPs), a measurable immune response that is a proxy for protection from infection, will only be unearthed when samples are available from successful placebo-controlled trials with thousands of volunteers. “There’s nothing we can measure in the blood that says, ‘This patient is vaccinated and now protected,’” says Nigel Curtis, a TB vaccine researcher at Murdoch Children’s Research Institute and the University of Melbourne.
Scientists have for years assumed that a strong response from T cells that eliminate infected cells was crucial to defending against TB. “A vast amount of research demonstrates a need for CD4+ T (also called TH1) cells,” says Mortensen, which seem to be “essential for infection control and preventing disseminated disease.” He notes, however, that more CD4+ T cells doesn’t necessarily mean better protection against TB, so their abundance is not a perfect COP. In recent years, T helper 17 (Th17) cells that produce proinflammatory cytokines have been described as protective, Mortensen adds.
TB and humans have been battling and sharpening their swords on each other for so long that the balance between who gets to win and who gets to lose is a really fine one.
—Thomas Scriba, University of Cape Town
To generate a vaccine that recruits and activates T cells well, McShane’s group at Oxford used a modified vaccinia Ankara (MVA) virus as a vector to deliver a TB antigen, 85A. In an early clinical trial, the resultant MVA85A vaccine “stimulated what we thought was the right kind of immune response,” recalls McShane—specifically, the shot induced CD4+ T cells that secrete interferon gamma, tumour necrosis factor, and interleukin-2. Nevertheless, in a trial of infants in South Africa who had already been vaccinated with BCG, MVA85A failed to show an improvement over BCG vaccination alone. “To everybody’s amazement, it was found that the vaccine didn’t protect against tuberculosis,” notes Curtis, who was not involved in the trial. For him, this suggested that “what we are measuring and what we think are protective are the wrong things.” The researchers concluded that the level of T cell response induced in the trial was not enough to boost protection after BCG.
“TB is a respiratory infection, and one problem is that we almost always end up studying what is going on in the blood,” says Dockrell, “but this does not give you insight into what is happening in the lung, where there are specialized cells” that may be crucial in the fight against TB.
TB Vaccines in the Pipeline Take Varied ApproachesBCG, or the Bacille Calmette-Guérin vaccine, elicits a multipronged immune response that effectively fends off tuberculosis in children in developing countries, where the disease is still common. But the vaccine’s protection wanes with age, and the pathogen can infect adolescents and adults, causing a lung disease characterized by a persistent, sometimes bloody cough. Researchers aren’t sure which parts of the immune response are most critical for protection to be effective, and are taking varied approaches to improve on BCG with next-generation TB vaccines. One leading candidate shown here involves the same microbe as BCG, but with a few genetic tweaks that researchers hope will provide better protection. Another takes an entirely different approach with manipulated antigens from the BCG bacterium. Still other TB vaccines in the pipeline include live and killed whole mycobacteria of various species—including Mycobacterium tuberculosis, which causes tuberculosis—and viral delivery of the genetic recipe for mycobacterial antigens. |
BCG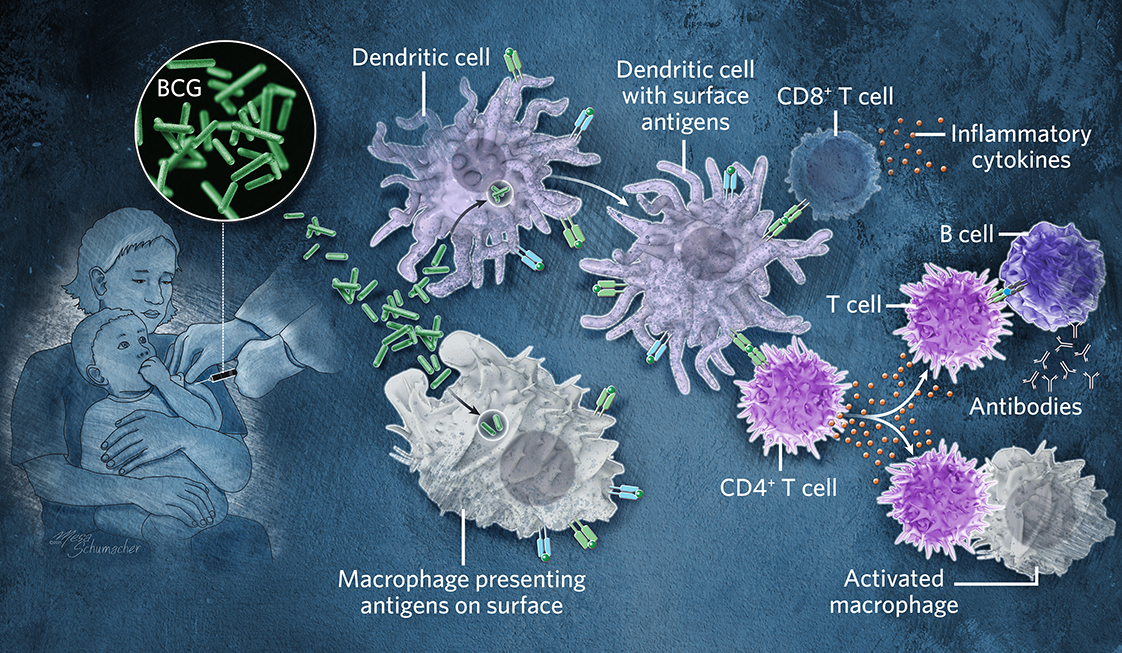 © Mesa Schumacher This 100-year-old vaccine is a live but weakened form of a cattle bacteria that is related to Mycobacterium tuberculosis (Mtb). Inoculation attracts frontline immune cells to the site of the injection. Dendritic cells and other antigen-presenting cells display parts of the BCG microbe on their surface to drive a response by T cells, which fight future infection with the pathogen and train B cells to produce antibodies. Both BCG and Mtb go into vesicles called phagosomes, where they interfere and stop their own destruction. BCG however is eventually degraded, whereas Mtb survives for a long period of time within cells. See full infographic: WEB |
Tuberculosis vaccines in the pipeline
There are more than a dozen TB vaccines in clinical trials. While new TB vaccines may not prevent a person from ever getting infected, some have shown signs that they could stop infected people from progressing to disease. “Stopping infection would be the Holy Grail, but it is a really, really high bar,” acknowledges Gordon. “Stopping people latently infected from progressing to disease would be a game changer.” In nearly all TB vaccine trials, volunteers had latent TB infection and prior BCG immunization.
Two vaccines, MTBVAC in Phase 2a and VPM1002 in Phase 3, are being trialed in adults as well as infants and neonates, which could be especially useful for immunocompromised children with HIV. This is noteworthy because most TB vaccine candidates are being trialed in adolescents and adults. “If we can stop adolescents and adults from getting TB, then we wouldn’t worry about children needing vaccines for TB,” says Scriba.
The candidate that seems to be on everyone’s lips is M72, a protein subunit vaccine being developed by GlaxoSmithKline (GSK). The vaccine includes a fusion of two proteins from M. tuberculosis and an adjuvant, AS01, which GSK uses in its blockbuster shingles vaccine and its leading malaria vaccine candidate. Although the M72 vaccine had not looked so promising in nonhuman primates, it lowered the incidence of pulmonary TB by 54 percent over three years in a recent trial involving more than 3,000 adults in Kenya, South Africa, and Zambia.
Because the vaccine is little more than a couple of proteins, “I was skeptical that this would work,” says trial investigator Robert Wilkinson, a TB researcher at the Francis Crick Institute in London. BCG is a live, replicating vector that “resembles the slower turnover of natural infection by M. tuberculosis,” Wilkinson explains, and while protection wanes in adolescence, BCG wards off TB throughout childhood. “My hunch was that such a type of vaccine would be best in TB: to provide a sufficiently lengthy period of immune stimulation to ward off natural infection. . . . In that respect I was proven wrong because protection [from M72] lasted for three years.”
Hawn, who is not involved in M72’s development, gives credit to the vaccine’s adjuvant, which triggers an innate immune receptor called toll-like receptor 4 (TLR4) to generate a robust immune response that calls in T cells in addition to antibody-generating B cells. “Historically, we just haven’t had that level of potency in adjuvants,” he notes. “We have new adjuvants, and our ability to trigger different immune responses in more nuanced ways has advanced a lot.”
Wilkinson notes that there were just 26 cases of TB in the placebo group and 13 in the vaccine group. He says a larger trial involving tens of thousands of people is the obvious next step before seeking emergency use authorization from a regulatory agency.
VPM1002 Whole-Cell Vaccine © Mesa Schumacher A genetically modified version of BCG is injected. Macrophages take up the bacterium into a phagosome, where it produces an enzyme that causes pores to form in the phagosome membrane. This allows antigens to leak out into the cytosol and trigger an inflammasome activation, in a similar way to Mtb, which BCG does not do. See full infographic: WEB |
Meanwhile, the Statens Serum Institut is developing two subunit vaccines, with its lead candidate H56 consisting of three antigens and a novel adjuvant in Phase 2b trials in Tanzania and South Africa. The institute is also developing a subunit vaccine called H107 with eight antigens specific to M. tuberculosis. Because these antigens are not shared by BCG, the H107 vaccine “does not cross react with BCG, which means that the two vaccines can be co-administered,” notes Mortensen, who is leading the development of H107.
I’m very excited about the mRNA vaccine technologies and hope it won’t take long before those are applied to TB, because they are clearly very nimble and extremely immunogenic.
—Thomas Scriba, University of Cape Town
Other protein subunit vaccines with adjuvants that had looked promising in early and mid-stage trials have failed, and this has turned off Andreas Kupz, a vaccine scientist at James Cook University in Queensland, Australia, to this approach. “As a whole, there was disappointment around these vaccines.” Kupz says he sees more hope in live vaccines that follow the approach of the original BCG. For instance, the Bill & Melinda Gates Medical Research Institute is conducting a trial that involves administering a booster dose of BCG to people aged 10 to 18 years in South Africa. This approach “has previously been considered ineffective,” Mortensen says, but the Gates trial showed that in South African adolescents that it could prevent 45 percent of sustained TB infections, or infections that lasted for more than six months. “It didn’t prevent them developing a positive blood test [for TB], which we think means they got infected,” explains immunologist Hazel Dockrell of the London School of Hygiene & Tropical Medicine, “but more of them reverted back to negative, which suggests they cleared the infection.”
Several new live vaccines are in the pipeline, and one of the most advanced is VPM1002, a genetically modified BCG currently in Phase 3 testing that was developed as part of a project spearheaded by Stefan Kaufmann at the Max Planck Institute for Infection Biology in Berlin. “We thought that BCG wasn’t bad at all,” says Kaufmann, “but we would like to improve it for neonates, [and] ideally also for use in adults and adolescents.” Among other improvements they made to the vaccine, Kaufmann and his colleagues added the gene for a pore-forming protein (listeriolysin O) from Lysteria. Once a bacterium is engulfed by a frontline immune cell such as a macrophage, it gets sequestered into compartments called phagosomes; listeriolysin O perforates the phagosomal membranes to allow leakage of VPM1002-derived molecules, which are presented on the cell’s surface to induce CD8+ T cells to attack infected cells, and the escape of DNA, which sets off proinflammatory pathways.
M72 Protein Subunit Vaccine © Mesa Schumacher Two recombinant proteins from the Mycobacterium bacteria that constitute the BCG vaccine are fused together and injected. Fusion proteins are taken up by immune cells that then display them on their surfaces to trigger an immune response against the antigens. A proprietary GlaxoSmithKline adjuvant (AS01) boosts that immune response. See full infographic: WEB |
Another live vaccine candidate differs from BCG even more by using M. tuberculosis itself. “There’s a belief that if you can move more closely to simulate the natural infection, that that might be a route to success,” says Wilkinson. The researchers at the University of Zaragoza in Spain who developed the so-called MTBVAC vaccine deleted two virulence genes to make M. tuberculosis safer and are now testing the modified bacterium in Phase 2a trials. “MTBVAC is likely to mimic a TB infection much closer than BCG, and induce a more similar memory immune response that is more likely to be protective,” Mihai Netea, an immunologist at Radboud University Nijmegen in the Netherlands who has been involved in studying MTBVAC’s immunological properties in the last few years, notes by email.
Scriba strikes a note of caution: given that the higher amounts of mycobacteria nearer the equator seem to push down efficacy of BCG for adults, the same phenomenon could repeat itself for these newer live mycobacteria vaccines. “There’s pretty good evidence that immune responses to environmental mycobacteria interfere with the efficacy of BCG. The question is, to what degree will that be an issue for MTBVAC and VPM1002?” Scriba says. “It will be interesting to see what happens when these vaccines are given to older individuals” who have a lifetime of exposure to mycobacteria. If cross-reactivity among mycobacteria does prove problematic, protein subunit vaccines or vector-based jabs such as those McShane’s group is now pursuing may turn out to be the better option. “You don’t want to put all your eggs in the one basket,” says Scriba.
Putting an end to tuberculosis
To reinvigorate the TB vaccine landscape, researchers must consider that BCG doesn’t just help prevent tuberculosis, it also primes the immune system to defend against respiratory diseases more broadly and even sepsis. Researchers observed this nonspecific protection soon after BCG was widely administered, when vaccination reduced not just TB deaths in children, but deaths from other causes as well. BCG is now also used as an immunotherapy for treating early-stage bladder cancer, put directly into the bladder to trigger a patient’s immune system to attack tumors. The related VPM1002 vaccine has also shown promise in clinical trials as a bladder cancer therapy.
In the last decade, researchers have steadily unveiled a mechanism for these nonspecific effects, finding that it involves genetic rewiring of innate immune cells, a phenomenon called trained immunity. “Essentially, people now believe that by giving BCG, you imprint a memory epigenetically for these cells to respond to other subsequent infections,” Kupz explains. Some scientists even speculate that this general mechanism of immune defense could explain most or all of the protection BCG provides against TB. While most of the TB vaccines in development are being trialed against tuberculosis specifically, Curtis is currently leading a multinational trial of medical workers who have received BCG to see if it has an influence on SARS-CoV-2 infection.
See “How Some Vaccines Protect Against More than Their Targets”
For now, TB vaccine development is being complicated by the COVID-19 pandemic. Experts who spoke with The Scientist note that some TB vaccine trials have been delayed in starting or slowed down. “It has definitely set us back, because we would have finished this trial [with M72],” Wilkinson says. Moreover, it is likely that cases of TB have gone unreported and that treatment has lagged during the pandemic, McShane adds. “Global lockdowns, focused on another pathogen, has disrupted TB control programs.”
Indeed, nine of the countries with the most TB cases saw a drastic decline, ranging from 16 percent to 41 percent, in diagnosis and treatment of TB infections in 2020, according to the NGO Stop TB Partnership. The organization estimated that 12 months of COVID-19 had eliminated 12 years of progress in the global fight against TB. Case numbers and deaths from TB will probably be officially lower for 2020 due to pandemic disruptions, says Kaufmann, “but TB may kill more people than COVID-19 in 2020 because those undiagnosed with TB will not be treated.”
More than 95 percent of TB deaths occur in low- to middle-income countries. A study from the London School of Hygiene & Tropical Medicine estimated that basic social supports could cut TB burden by 85 percent. “We are well adapted to survive it,” says Scriba, “but poor living conditions, poverty, overcrowding, being immunocompromised due to HIV—these lead to more disease.” The World Health Organization says there is a $3.3 billion resource gap for implementing existing TB interventions.
A vaccine can be a crucial part of a global strategy to tackle the scourge of TB infection and death. “If we have a vaccine that could stop [recipients] developing TB disease from a latent infection or, even better, eradicate that latent infection, that would be phenomenal,” says McShane.
While the pandemic has temporarily hindered vaccine development, there is hope that there will be some cross-fertilization from the intense work that went into the COVID-19 vaccines, even if TB is seen as a tougher foe. “COVID is an easier target for a vaccine. TB is much more complex,” acknowledges McShane. Still, “we need to try and see what lessons can be learnt to try and move TB along quicker.” Some researchers suggest that the mRNA vaccine strategy, which boasts strong stimulatory effects on the immune system, could be adapted to target TB, for example. “I’m very excited about the mRNA vaccine technologies and hope it won’t take long before those are applied to TB, because they are clearly very nimble and extremely immunogenic,” says Scriba.
Wherever the answer lies, researchers in the field are determined to find it, with clinical programs forging ahead. “This is the biggest killer amongst all infectious diseases,” says Kupz. “We need a better vaccine for TB.”
Vaccine DeliveryIn the 1920s, BCG was administered orally. Doctors soon switched to giving an injection just under the skin, and this has remained standard practice for nearly a century. Now, though, researchers are rethinking delivery, with entry into the lung and intravenous injections being considered. “We’re working on delivering vaccines into the lungs, because that’s how TB enters the body,” says the University of Oxford’s Helen McShane. “And there’s some animal data to show that that’s the best way to protect.” She says her studies point to more-robust immune responses in the lungs and the blood when a TB vaccine is given as an aerosol. “When BCG is given directly into the lung, intranasally or by spray, resident memory cells become active,” says vaccine scientist Andreas Kupz at James Cook University in Queensland, Australia. These are localized memory T cells and possibly memory B cells that are prepped to respond to the reappearance of a specific pathogen. These cells also seem to be stimulated by IV infusion of BCG. A recent study in Nature reported better CD4+ and CD8+ T cell responses in rhesus macaques that were immunized intravenously with BCG, compared to monkeys that received intranasal and intradermal administration. “The protection conferred by IV BCG can teach us more about what kind of immune response is needed, and then we can design other vaccines that induce that response,” says McShane. |








