 © ISTOCK.COM/CREATIVE_OUTLETIn 2015, biopharmaceutical company Juno Therapeutics launched a Phase 2 trial testing a therapy for adult relapsed and refractory acute lymphoblastic leukemia (ALL), a blood cancer that, with current treatments, only 10 percent of patients survive past five years. Developed in collaboration with researchers at Memorial Sloan Kettering Cancer Center, Juno’s chimeric antigen receptor (CAR) T-cell therapy JCAR015 was engineered with a specific protein to help the immune cells recognize, and selectively kill, tumor cells displaying the CD19 antigen on their surface. Like other CAR T-cell products in development, the therapy had shown tantalizing potential, achieving remission in patients for whom other treatments had failed.
© ISTOCK.COM/CREATIVE_OUTLETIn 2015, biopharmaceutical company Juno Therapeutics launched a Phase 2 trial testing a therapy for adult relapsed and refractory acute lymphoblastic leukemia (ALL), a blood cancer that, with current treatments, only 10 percent of patients survive past five years. Developed in collaboration with researchers at Memorial Sloan Kettering Cancer Center, Juno’s chimeric antigen receptor (CAR) T-cell therapy JCAR015 was engineered with a specific protein to help the immune cells recognize, and selectively kill, tumor cells displaying the CD19 antigen on their surface. Like other CAR T-cell products in development, the therapy had shown tantalizing potential, achieving remission in patients for whom other treatments had failed.
See “The CAR T-Cell Race”
But in May 2016, things started to go terribly wrong; one of the 68 patients being treated with JCAR015 died from cerebral edema, a swelling of the brain. Then in July, another two patients died...
The fatalities, widely reported and discussed, came as a blow to the field, with some investors and health care analysts questioning whether the company—and the FDA—had acted responsibly. By early 2017, Juno’s shares had sunk to less than half of their value the previous summer, and in March, the company decided to call it quits on JCAR015 altogether, although it plans to continue development on other CD19 therapies.
JCAR015 is not the first CAR T-cell therapy to have been associated with patient deaths. In fact, even those trials considered a success sometimes have troubling safety profiles. For example, Novartis’s lead candidate, the CD19-targeting CLT019, demonstrated a remarkable 82 percent remission rate in a 2016 trial with 50 children and young adults with ALL, but the treatment caused severe cytokine release syndrome (CRS)—a potentially life-threatening condition where large volumes of T cell–released cytokines trigger inflammation and fever—in nearly half of patients. And in September, Santa Monica, California–based Kite Pharma reported serious neurological side effects in one-third of its patients and CRS in one-fifth, as well as two patient deaths, during a trial of its lead CD19-targeting candidate KTE-C19, a therapy designed to treat non-Hodgkin lymphoma.
Although administration of steroids or antibodies targeting T-cell receptors can mitigate these side effects during treatment, concern over patient safety has grown to become “the theme of the field,” says Sean McCarthy, CEO of CytomX Therapeutics, a San Francisco–based biotech developing CAR-based cancer therapies. “In general, in oncology, a higher level of toxicity has been accommodated by both physicians and patients,” he adds, “but there’s a limit.”
Nevertheless, researchers remain excited about the promise of CAR T-cell therapies to fight many treatment-resistant cancers. (See “Resist or Desist” on page 40.) Even in the recent Juno trial, “many of the patients who were treated had really great responses,” says Marcela Maus, director of cellular immunotherapy for cancer at Massachusetts General Hospital. And in late February, Kite announced that one-third of patients who’d received KTE-C19 showed no detectable cancer after six months.
As Novartis and Kite push for regulatory approval of their CD19-targeting CAR T-cell therapies this year, the field is striving to increase safety without sacrificing the treatments’ ability to beat cancer. “These therapies are so powerful and have so much potential, we have to find a way to manage the toxicity,” says Maus. “I would certainly not want to put the brakes on everything and say we have to go back to all animal models before we can go on.”
Emergency brakes
While all CAR T-cell therapies tested thus far cause adverse reactions in at least some patients, certain treatments show greater toxicity than others, and understanding why has proven challenging. Different companies use different manufacturing processes, clinical protocols, patient populations, and dosing regimens, making the identification of specific risk factors difficult. To help disentangle these issues, the FDA recently proposed establishing central databases to keep track of safety indications for CD19-targeting CAR T-cell therapies such as those being advanced by Juno, Kite, and Novartis. The hope is that combining trials’ often small data sets could help researchers identify particular steps in development or administration that are linked to increased risks.
In the meantime, investigators are taking steps to improve patient safety during ongoing trials. “I think this point is being stressed by all companies at the moment,” says Ronald Dudek, founder of early-stage CAR T-cell company Living Pharma and a consultant on CAR T-cell therapy development. One approach, he says, is simply getting better at recognizing common side effects and responding appropriately. “I look forward to some standardization of CRS monitoring technologies and some bona fide diagnostics that might help catch the syndrome at an earlier stage.”
With this sort of real-time trial management in mind, several companies are investigating ways to mediate the action of CAR T cells during treatment, to rein in side effects before they become serious. A standard measure is to administer a general immunosuppressive drug such as tocilizumab to reduce inflammation—a method Dudek calls “the sledgehammer approach”—but researchers are now developing more sophisticated technologies to exercise finer control when hitting the immunological brakes.
In 2014, Houston-based Bellicum Pharmaceuticals pioneered a “suicide switch,” CaspaCIDe, which can be engineered into T cells. The switch comprises an enzyme involved in programmed cell death (the gene for which is transduced into patients’ T cells ex vivo), along with a small molecule activator, rimiducid. In the event of severe side effects in a patient, clinicians can administer rimiducid, triggering self-destruction of the modified T cells in as little as 30 minutes. The technology is incorporated in the company’s lead candidate, BX-501, a therapy using partially matched donor T cells (from a parent, for example) that is currently being evaluated in children with blood disorders ranging from leukemia to sickle cell disease.
Other companies are exploring variations on the theme. Cellectis and Juno are both trialing treatments containing T-cell safety switches that rely on antibodies to trigger cell death. And Ziopharm Oncology has recently developed technology to tamp down the activity of the CAR T cells without killing them entirely, leaving open the possibility of reactivating the treatment if the patient is able.
“First-generation products didn’t have switches,” says Eric Ostertag, CEO at gene and cell therapy company Poseida Therapeutics. “But just about everyone I’m aware of is moving in that direction.”
Engineering safer therapies
 LOCKED AND LOADED: T cells engineered to carry chimeric antigen receptors (CARs) on their surface can bind to tumor-specific antigens to target the cells for destruction. © LUCY READING-IKKANDAWhile managing CAR T-cell therapy toxicity could help keep already-designed treatments on their march to the clinic, many immunotherapy companies are also working to develop a new generation of therapies that are inherently safer, yet just as efficacious. “The goal is to separate the toxicity from the antitumor efficacy,” says Dudek. “That’s sort of the holy grail in this space.”
LOCKED AND LOADED: T cells engineered to carry chimeric antigen receptors (CARs) on their surface can bind to tumor-specific antigens to target the cells for destruction. © LUCY READING-IKKANDAWhile managing CAR T-cell therapy toxicity could help keep already-designed treatments on their march to the clinic, many immunotherapy companies are also working to develop a new generation of therapies that are inherently safer, yet just as efficacious. “The goal is to separate the toxicity from the antitumor efficacy,” says Dudek. “That’s sort of the holy grail in this space.”
A key part of achieving this goal will be improving CAR specificity for target cells. With current therapies, the destruction of normal cells is often an unavoidable side effect when healthy tissue carries the same antigens as tumors; noncancerous B cells, for example, are often casualties in CD19-targeted therapies. While cell damage can be managed to an extent, these “on-target, off-tumor effects” can be fatal—particularly in solid tumors, where T cells are more likely to encounter target antigens on healthy as well as cancerous tissue. “The prediction is that it’s going to be very difficult to treat solid-tumor patients with CAR T [cells], unless we can find antigens that are exquisitely localized to cancer tissue and not present at all on normal tissue,” says McCarthy. “The reality is that there are very few, if any, such targets.”
One approach to improving specificity is to engineer CARs with not one, but two antigen-binding domains. The resulting bispecific CARs could reduce off-target effects by requiring that two tumor antigens are present—or that one tumor antigen is present and a second, healthy-cell antigen is absent—before T-cell activity is stimulated. Such approaches have shown improved specificity in preclinical models of prostate cancer, and Juno states it has been developing bispecific technologies over the last couple of years.
Signals in the tumor microenvironment could also be exploited to help CAR T cells distinguish cancerous from healthy tissue. In January, Cellectis published a method to engineer CARs with oxygen-sensitive domains that render T cells ineffective unless they’re in a hypoxic environment—a characteristic of up to 50 percent of solid tumors (Sci Rep, 7:39833, 2017). And CytomX’s Probody technology masks the target-binding region of an antibody until it is broken down by proteases unique to the tumor microenvironment. “The technology is really designed to avoid binding to normal tissue,” explains McCarthy. “Concentrating the active antibody in tumor tissue allows us to [lengthen] the therapeutic window, or create a therapeutic window where there may not be one.”
Efficacy boost
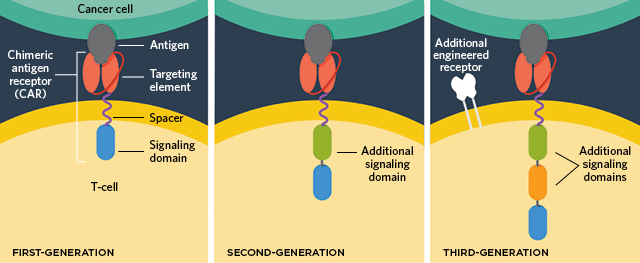 IMPROVING CAR T CELLS: In addition to a specific cancer-targeting antibody, a transmembrane component, and a signaling domain that amplifies the activation of the T cells (left), new CAR T-cell technologies have added additional costimulatory domains within the cells (middle), engineered receptors (right), and even safety switches (not pictured) to improve targeting of the T-cell attack and minimize side effects.© LUCY READING-IKKANDA
IMPROVING CAR T CELLS: In addition to a specific cancer-targeting antibody, a transmembrane component, and a signaling domain that amplifies the activation of the T cells (left), new CAR T-cell technologies have added additional costimulatory domains within the cells (middle), engineered receptors (right), and even safety switches (not pictured) to improve targeting of the T-cell attack and minimize side effects.© LUCY READING-IKKANDA
Of course, as CAR T-cell therapies progress in development, it’s not just safety profiles that researchers hope to improve; scientists also aim to further enhance the therapies’ efficacy. Tweaking the genetics of T cells beyond adding CARs is a broad approach drawing more attention, reflected in several deals between CAR T-cell developers and biotechs employing the genome-editing tool CRISPR-Cas9. In 2015, Novartis announced a five-year collaboration with Intellia Therapeutics; soon after, Juno entered a five-year partnership with Editas Medicine. “What you’re seeing is a logical marriage of gene-editing technologies to CAR T cells,” says Dudek. “Companies have announced they’re working on better-designed cells that work optimally in the hostile tumor microenvironment.”
One application for genome editing is the deletion of genes that can dampen T cells’ ability to fight tumors—a possibility being explored as part of the first US trial to test CRISPR’s potential in humans, scheduled for early 2017. Designed by researchers at the University of Pennsylvania, the trial will include disrupting a gene coding the immune checkpoint protein PD-1, a T-cell surface receptor that tumors often exploit to dampen T-cell activity. While the protein can be targeted with inhibitors in the clinic, knocking it out genetically could increase the persistence of T-cell activity—though the approach deserves caution, notes Dudek. “Knocking out PD-1 might seem like a great idea, but you’re taking the brakes off a very powerful locomotive, so you’d better be able to stop it if something goes wrong.”
The precision of CRISPR could one day also be used to improve the delivery of the CAR genes themselves. Earlier this year, researchers at Memorial Sloan Kettering Cancer Center showed that, unlike viral delivery—which inserts the CAR gene randomly into T-cell DNA—CRISPR-mediated delivery can introduce a CAR gene at a specific location in the genome. T-cells created using this method showed higher potency, the researchers reported, and outperformed traditional CAR T cells in mice with ALL (Nature, doi:10.1038/nature21405, 2017).
Longer term, some companies are eyeing the possibility of moving beyond patient-specific products altogether, exploring the production of “off-the-shelf” CAR T cells from donated T cells. This approach has the potential to generate T-cell populations to be used in thousands of patients with lower risk of graft-versus-host disease, in which the recipient’s immune system attacks the donor cells. Last year, Kite joined forces with the University of California, Los Angeles, to investigate relevant methods, while Juno partner Fate Therapeutics paired up with Sloan Kettering; just this February, Cellectis received approval from the FDA to begin Phase 1 trials of an off-the-shelf therapy for two types of blood cancer.
For now, while Juno scrutinizes its pipeline, all eyes are on CAR T-cell therapy frontrunners Kite and Novartis. If one or both of these companies’ products gain approval in 2017—a prospect deemed likely by market analysts—“it’s going to raise every CAR T cell’s boat,” notes Dudek, “as well as make this emerging field more real.” Maus agrees: “It’s going to be so exciting when the first cell therapy gets FDA approved,” she says. “That’s going to be a significant milestone.”
And with such a variety of technologies trailing close behind, it’s little wonder that the field remains optimistic, in spite of recent setbacks. “Patient deaths in clinical trials are of course the last thing we want to see,” says CytomX’s McCarthy. “But often, what we learn from these setbacks actually allows us then to move forward. I personally remain hugely optimistic for all these approaches in cancer immunotherapy, and I think that the best is very much yet to come.”
Interested in reading more?





