ABOVE: © shutterstock.com, Kateryna Kon
In 2007, Ho Man “Holly” Tang took a break from her undergraduate biology studies at Iowa State University to join her older brother, Ho Lam “Hogan” Tang, then a doctoral student at the Chinese University of Hong Kong, to work on a project together. In Ming-Chiu Fung’s immunology lab, Hogan had been investigating how disturbances in the cytoskeletons of cells might contribute to the fragmentation of mitochondria during apoptosis, the most familiar form of cell suicide. But the siblings had a more fundamental question: Can cells recover from the cellular chaos that ensues once apoptosis is initiated?
There are many different triggers of apoptosis, but they all ultimately activate executioners called caspases. Cleaving hundreds of different kinds of proteins within a cell, these enzymes wreak havoc on the genome, attack structural proteins composing the cell’s organelles, and dismantle the cytoskeleton, leading cells to...
The Tangs exposed a variety of human cancer cells to toxins such as ethanol and waited for long-established signs of apoptosis, such as caspase activation and cell shrinking. Next, instead of throwing away the “dead” cells at the end of the experiment, “we washed and incubated them with fresh medium overnight,” says Tang.
It doesn’t seem to matter how apoptosis started. If good conditions are restored, anastasis can rescue cells following a variety of insults.
“Surprisingly, some cells had regained normal morphology when we looked at them the following morning.”1 The siblings named this phenomenon anastasis. Apoptosis means “falling” in Greek, and the process facilitates the natural turnover of cells, akin to petals falling from a fading flower or leaves from a tree in autumn. Anastasis, on the other hand, means “rising,” and in Christianity refers to the resurrection of Jesus.
The research community was initially skeptical that cells could come back from the dead. “Our finding was quite controversial—one of our first few papers got rejected more than 11 times in three years,” says Tang, now a molecular and genetic biologist at Johns Hopkins University.
He got more experimental support for anastasis after he joined the lab of Denise Montell, a cell and developmental biologist then at Johns Hopkins. In collaboration with his new lab, former colleagues in China, and his sister, who had followed him to Baltimore after completing her master’s degree at Iowa State, Tang conducted experiments not only on human cancer cell lines, such as HeLa, but also on normal, cultured mouse liver, rat heart, and ferret brain cells, as well as multiple cultured human cells, including fibroblasts.2 Time and again, the cells appeared to recover from late-stage apoptosis, even after suffering DNA damage and cell fragmentation. “The moment I really knew there was an important phenomenon was when we made a movie of an entire field of human lung cancer cells shriveling up and blebbing and then recovering,” says Montell, now at the University of California, Santa Barbara. “It was striking.”
Since 2012, when anastasis was formally named, researchers have delved deeper into precisely how cells apparently can stitch themselves back together after being badly damaged by apoptosis. Scientists have witnessed fragmented mitochondria in cultured human cells become whole again, and caches of mRNAs created and held in reserve during apoptosis to help jump-start cells back to life. They have also found evidence that anastasis can occur in vivo in Drosophila.
“We had always looked at apoptosis as black and white, all or nothing, so it’s really fascinating that research is now finding otherwise,” says Stephen Tait, a cell biologist at the University of Glasgow. “You can get caspase activation cleaving all these proteins in a way that seems incompatible with life, yet the cell somehow survives.”
The revelation might change how scientists define life and death on the cellular level, adds Yinan Gong, an immunologist and cell biologist at the University of Pittsburgh Medical Center. “What does death mean to a cell?”
How cells survive

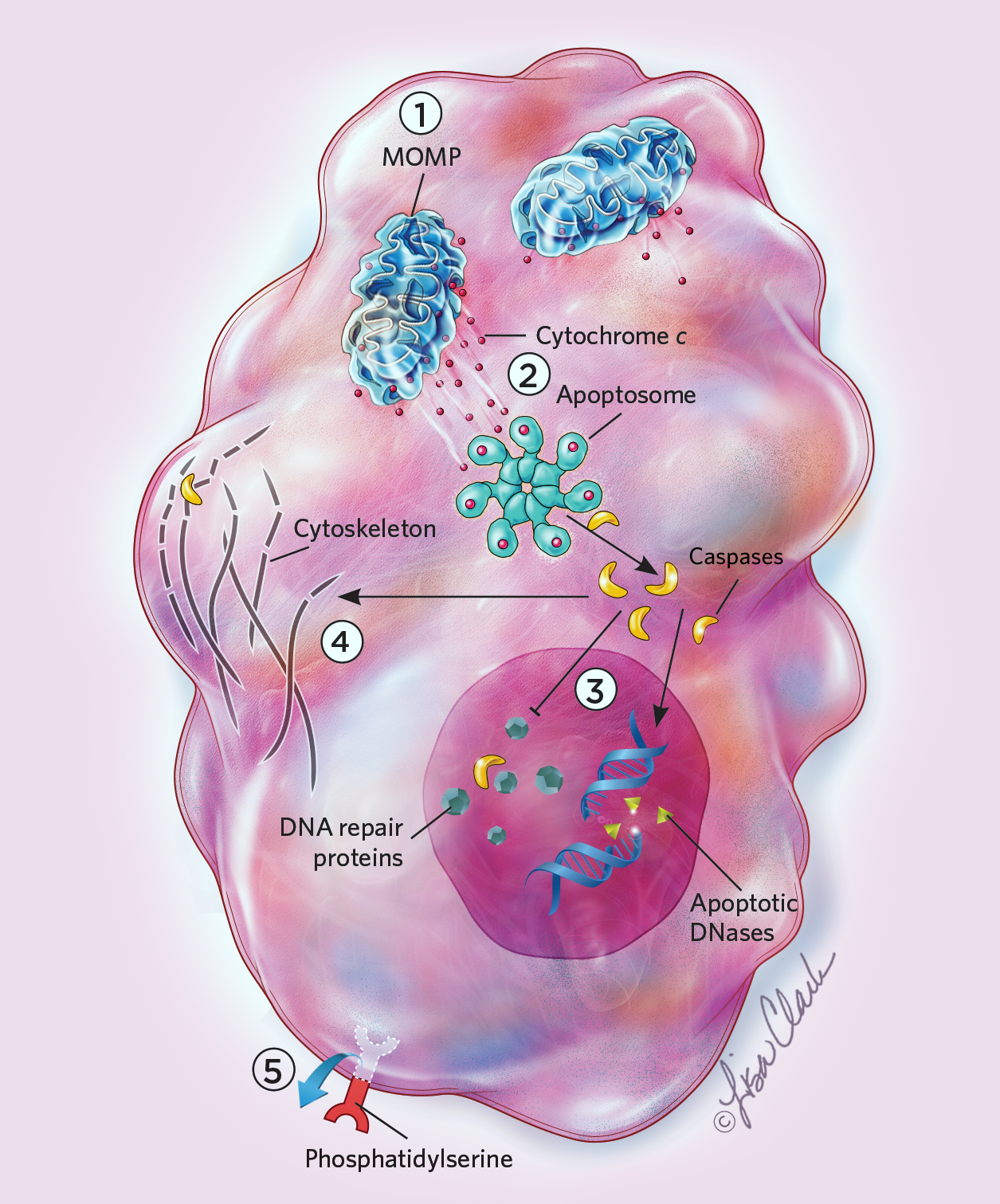
Cells can live for days, months, even years, but once apoptosis starts, it takes no time at all for death to set in. Within 10 minutes of caspase activation, the cell visibly transforms: its membrane distorts as the cell shrinks, and its DNA condenses into bundles that then break apart. Caspases also cause a molecule known as phosphatidylserine to flip from the inner to the outer surface of the cell membrane, serving as an “eat me” signal for phagocytic cells. (See illustration.) “You activate these caspases like hired killers, which cleave maybe several hundred substrates in cells and kill cells within five to ten minutes,” says Gabriel Ichim, a cell biologist at the University of Lyon in France.
Apoptosis is triggered many different ways, but they all ultimately activate enzymes known as caspases that disrupt a cell’s DNA, organelles, and cytoskeleton. Caspases also recruit other cells to eat the dying cell’s remains. Even after commencing this suicidal process, cells can recover through a recently discovered process dubbed anastasis. But if anastasis happens late in apoptosis, the surviving cells may carry major chromosomal scars and other genetic defects that can lead to malignancy.
In 2015, working with cultured human cells, Ichim, Tait, and their colleagues described a phenomenon similar to but distinct from anastasis known as “failed apoptosis,” in which cells recover after only partially undergoing the radical changes that normally occur during apoptosis.3 It was previously thought that when a cell’s mitochondria began leaking cytochrome c, a potent activator of caspases, all of the organelles did so in sync throughout the cell. However, when the researchers checked, they found that sometimes only a few of a cell’s mitochondria leak, meaning only a small fraction of caspases are activated, and the cells survive.3
Like anastasis, failed apoptosis shows that the initiation of apoptosis is not the death sentence it is often thought to be, says Tait. But failed apoptosis involves cells that only partially go through apoptosis—the levels of active caspases are much lower than when apoptosis is successful, and only a limited number of the enzymes’ key targets are cleaved. In contrast, the Tangs’ findings suggest that cells can even recover when apoptosis is full-blown, Tait says.
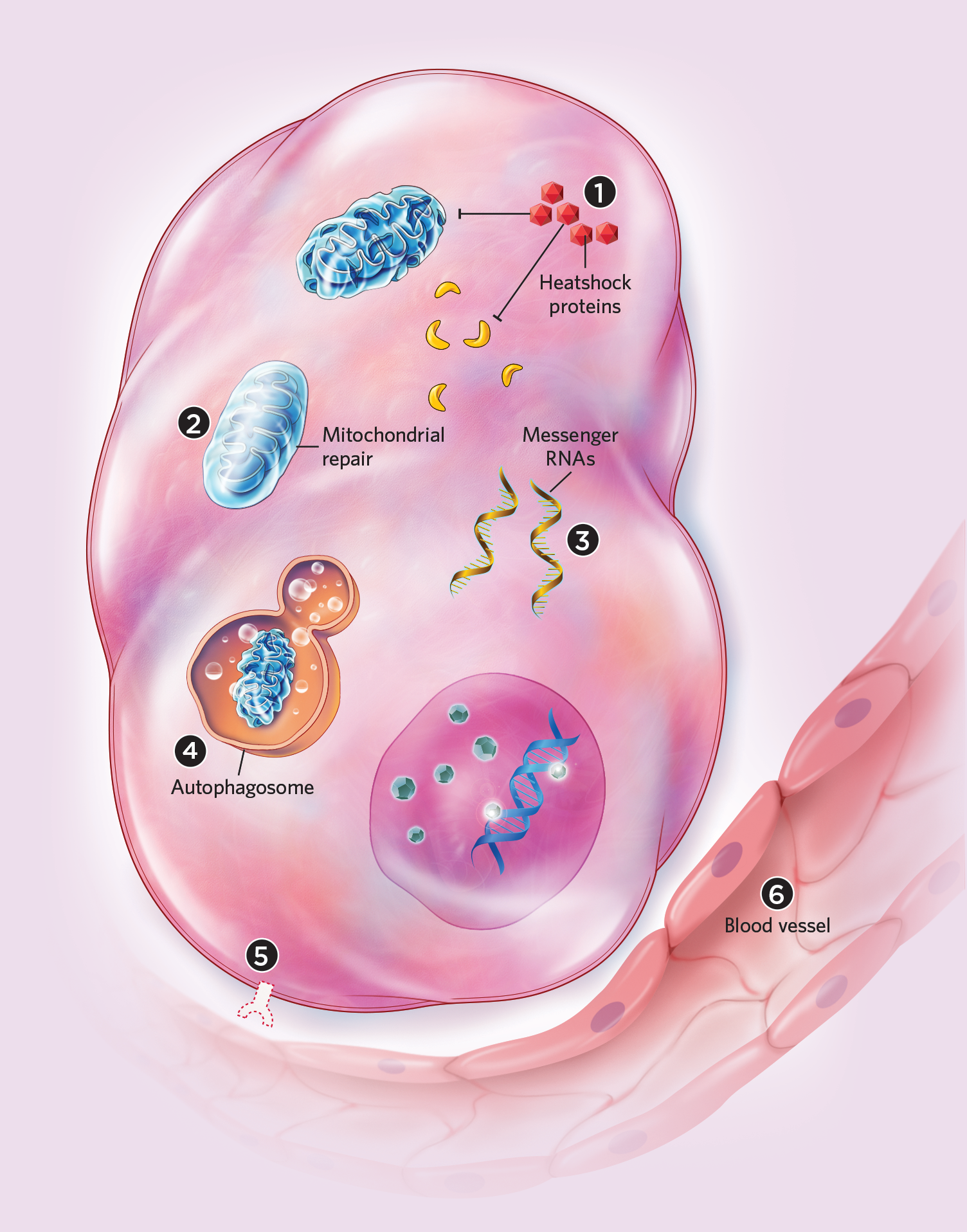
The Tangs and their colleagues have found, for example, that anastasis might permit recovery from apoptosis even after cells have begun to break apart. Earlier this year, the researchers published time-lapse live-cell microscopy showing HeLa cell fragments reassembling into an apparently normal morphology.4 It doesn’t seem to matter how apoptosis starts; if favorable conditions are restored, anastasis can rescue cells following a variety of insults, including cold shock, protein starvation, and exposure to toxic chemicals. In cells that have advanced to late-stage apoptosis, fragmented mitochondria can even fuse and regain their normal structures, the Tangs and their colleagues have found.4,5 Recovering cells also lose the “eat me” signal from their surfaces after only a few hours, thus escaping phagocytosis.2
In 2017, Montell and the Tangs independently published deeper dives into the molecular signatures of anastasis. Montell’s group found that more than 1,000 genes were upregulated in apoptosing HeLa cells given fresh media, and that the molecular responses of anastasis were similar to some extent to those in cells recovering from autophagy, the cellular process by which proteins and organelles are recycled.6 In addition, mRNAs linked to cell rescue apparently accumulated before apoptosis was arrested, suggesting that cells prepare for anastasis early in apoptosis to support a potential recovery. During anastasis in mouse primary liver cells, the Tangs and their colleagues noted striking changes in the expression of genes involved in the removal of damaged mitochondria, injurious free radicals, and other cellular components, as well as the arresting of the cell cycle, perhaps to give the cell time to repair.7
See “Eat Yourself to Live: Autophagy’s Role in Health and Disease”
The take-home message is clear, says Tait: cell death is not clear-cut. “There’s this idea that either a cell is dead or it’s not, but I certainly think there may be a lot of grays in between.”
Validating anastasis
As much as scientists in the past decade have suggested that apoptosis is not always final, there is still widespread skepticism regarding these results. One reason anastasis remains controversial is the question of whether or not cells seen alive after exposure to apoptosis-inducing stressors actually experienced caspase activation, or whether they avoided the stressor that would have activated the executioners. Cells that undergo anastasis can also be indistinguishable from surrounding healthy cells. “It’s a real challenge to track these surviving cells in the long term, especially in vivo,” Ichim says.
To monitor cell survival in vivo after apoptosis is initiated, the Tangs and Montell independently developed gene constructs—CaspaseTracker7 in 2015 and CasExpress8 in 2016, respectively—that generate fluorescent proteins during caspase activation, and then fluoresce another color indefinitely thereafter. Single-cell and time-lapse live-cell microscopy then tracks cell fate.
This approach helped the teams observe anastasis in vivo for the first time. Using these constructs, the Tangs and Montell have seen cells in multiple types of tissues in Drosophila embryos experience caspase activation before recovering completely after the appearance of apoptosis’s usual hallmarks, such as cell shrinking and membrane blebbing. The researchers are now working to see if these findings hold true in rodents.
The results are still not widely accepted, in part because previous research has shown that caspases contribute to processes other than cell death. For instance, in the nervous system, caspases are activated during neuronal pruning related to learning and memory, notes Scott Dixon, a chemical biologist at Stanford University, so caspase activation may not be a definitive marker for apoptosis.
Montell and her colleagues note that one way to distinguish anastasis activity from other goings-on involving caspases is that non-apoptotic caspase activation typically happens in specific stages of development and in every cell of the same type in a tissue. In contrast, during development, anastasis can occur in random fractions of cells sporadically over time.10 Montell agrees that more research is needed to understand what exactly is taking place. Assuming cells can under some circumstances bounce back from apoptosis and other seemingly fatal processes, it is still unclear how general or important such recovery is. “It is a new field,” says cell and molecular biologist Shoukat Dedhar at the University of British Columbia. In addition to validating the early findings put forth by the Tangs, Montell, and others, he adds, researchers in the field must still demonstrate the relevance of cell death recovery.
Anastasis: How Cells Cheat DeathApoptosis is triggered many different ways, but they all ultimately activate enzymes known as caspases that disrupt a cell’s DNA, organelles, and cytoskeleton. Caspases also recruit other cells to eat the dying cell’s remains. Even after commencing this suicidal process, cells can recover through a recently discovered process dubbed anastasis. But if anastasis happens late in apoptosis, the surviving cells may carry major chromosomal scars and other genetic defects that can lead to malignancy. Apoptosis
|
Anatasis
|
Effects of apoptosis recovery
One possible function of anastasis is as a cellular survival mechanism, posits Tang, limiting the permanent damage that would occur in response to a temporary situation. For instance, tissues in a developing organism might randomly experience transient shortages of growth factors, an event that could trigger apoptosis at a time when cells are proliferating at a rapid rate. Montell and her colleagues found that anastasis can happen during development in Drosophila, with cells surviving caspase activation in the larval brain and in the imaginal discs that, during metamorphosis, develop into adult limbs and organs.10
Anastasis may also promote evolutionary change, Tang says. “Our recent studies demonstrated the occurrence of anastasis in germ cells of Drosophila after transient exposure to physiological and environmental stresses such as starvation or cold shock,” he notes.9 This raises the possibility that these cells might acquire new mutations generated during apoptosis that could be passed on to progeny.
Anastasis might serve more purposes than simple protection. In 2012, the Tangs and Montell found that while cells rescued from apoptosis could repair genetic damage, sometimes there were errors in the way they knit their genomes back together. As a result, a percentage of the surviving cells had chromosomal abnormalities and other genetic defects that led to malignant growth. This suggests that repeatedly bringing cells to the brink of death might explain the higher risk of cancer in tissues exposed to repeated assaults, such as liver cancer due to alcoholism and oral, esophageal, or stomach cancer that can result from the regular consumption of very hot beverages.7 Even with failed apoptosis, where damage is much less severe before recovery, cells’ genomes sometimes become unstable, and some of the cells even turn malignant, according to research conducted by Tait, Ichim, and their colleagues.3 “We’re following cells to see if there are long-term consequences for cancer progression, and we’re finding they can experience genomic instability,” says Ichim. “They can be more aggressive, and form bigger tumors in mice.”
The revelation might change how scientists define life and death on the cellular level.
Cells’ ability to recover from programmed cell death might also play a role in some cancers’ ability to evade therapeutic approaches. Radiation and many commonly used chemotherapy drugs induce apoptosis, and while this may kill most tumor cells, any that survive might result in relapse and metastasis.9 Moreover, cancer cells that survive such therapies might conceivably develop new mutations that help them resist drugs, says Dedhar, who is currently searching for molecular pathways that are activated in cancer cells that “recovered from the brink of death.”
Tang and his colleagues have also found that anastasis can activate genes in Drosophila linked with angiogenesis and cell migration,7 processes that could enhance nutrient absorption and remove waste to help cells recover from apoptosis, he says. At the same time, these changes could enhance the spread of cancer cells.
Given the potential links between cancer and anastasis, interventions that target this process could help prevent the development of cancer, or treat it once it appears, Tang says. “Suppressing anastasis in dying cancer cells during and after cancer treatment could be a novel therapeutic strategy to cure cancers by inhibiting cancer relapse.”
Conversely, he notes, targeting anastasis could one day treat diseases that stem from the loss of unrenewable cells, such as cardiomyocytes or neurons. “Currently, one of the major efforts in my lab focuses on identifying key anastasis regulators and small molecules that promote or suppress anastasis,” Tang says. “Our findings will provide us a list of essential tools to control anastasis, thereby launching new fields of research for exploring the potential functions and therapeutic applications of anastasis.”
Ultimately, a better understanding of anastasis might not only help save cells from disease and injury, but also teach scientists more about cell death in general. “The Holy Grail question is whether we can predict the fates of cells at the single-cell level,” Gong says. “For instance, if we irradiate a tumor, we might know that maybe 90 percent of the tumor will shrink, but we can’t tell which 90 percent. Predicting which cells will die and in which way will ultimately help us minimize side effects and maximize beneficial effects. It’s cell death 2.0.”
The many faces of deathAnastasis is not the only way to grant cells a stay of execution. Last year, in experiments involving gene silencing in cultured mammalian cells, immunologist and cell biologist Yinan Gong of the University of Pittsburgh Medical Center and his colleagues unexpectedly found that another form of programmed cell death, necroptosis, can also reverse. In necroptosis—a programmed version of necrosis, a form of cell death linked with uncontrolled reactions to injuries or stress—a protein known as mixed lineage kinase-like (MLKL) opens holes in the plasma membrane, rupturing cells. Gong and his colleagues found that necroptosis does not always prove fatal. Instead, the ESCRT-III protein complex can isolate these holes onto bubbles in the plasma membrane. Shedding these bubbles then repairs the cells, a process the scientists dubbed “resuscitation.”10 The team hypothesizes that necroptosis happens when MLKL essentially overwhelms ESCRT-III. Other forms of cell death appear reversible, too. For example, in 2012, chemical biologist Scott Dixon of Stanford University found that ferroptosis, a form of cell death that is dependent on iron, can be reversed by treatment with lipophilic antioxidants or iron chelators. “Some people might assume that once a cell is exposed to a lethal stimulus, that’s it, there is no chance of recovery,” Dixon says. Now, research is showing “that a cell can be exposed to a stimulus that might be lethal, but within a period of time there is still a chance for an intervention to rescue the cell—not all hope is lost.” Even the striking form of cell death known as entosis, in which one cell swallows another alive, is reversible, with engulfed cells potentially emerging to continue living. “When a cell is engulfed, its nucleus can look fine, its plasma membrane can look fine, and with time-lapse video, we found it can get out and divide and do fine,” says Mike Overholtzer, a cell biologist at Memorial Sloan Kettering Cancer Center in New York. “Cells can even divide while engulfed and emerge and be perfectly viable.” He and his colleagues have found that entosis is prevalent in human cancers, and triggers for it include detachment from the extracellular matrix and glucose withdrawal,11 but what determines an engulfed cell’s fate is still unclear. All in all, these findings suggest that these pathways might not always be dead ends. “With people, when a doctor needs to call a death, it’s now well-defined—it’s brain death. We know that when patients get to a certain point, they cannot come back,” Gong says. “The question is still open as to what point a cell must reach before it does not come back.” EntosisIn entosis, one cell engulfs another living cell, which is then killed and digested by lysosomes. Sometimes engulfed cells survive, even proliferating within their cellular captor or escaping altogether.  © lisa clark NecroptosisNecroptosis is a programmed version of necrosis, a form of cell death linked with uncontrolled reactions to injuries or stress. The process involves the protein MLKL poking holes in the plasma membrane, which causes the cells to rupture. However, the cell can blunt this process through the ESCRT-III protein complex, which isolates these holes onto bubbles in the plasma membrane. Shedding these bubbles then repairs the cells, a process scientists dubbed “resuscitation.”  © lisa clark FerroptosisFerroptosis is a regulated form of cell death that is dependent on iron. Cells initiate this pathway when normal uptake and metabolism of the amino acid cysteine (cystine is the oxidized dimer form of cysteine) is disturbed. Once triggered, ferroptosis will result in cell death in a few hours. However, researchers can administer lipophilic antioxidants or iron chelators to completely protect cells from succumbing to this form of cell death. |
Charles Q. Choi is a freelance science writer living in New York.
References
- H.L. Tang et al., “Reversibility of apoptosis in cancer cells,” Br J Cancer, 100:118–22, 2009.
- H.L. Tang et al., “Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response,” Mol Biol Cell, 23:2240–52, 2012.
- G. Ichim et al., “Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death,” Mol Cell, 57:860–72, 2015.
- H.M. Tang et al., “Detecting anastasis in vivo by CaspaseTracker biosensor,” J Vis Exp, 132:e54107, 2018.
- H.M. Tang et al., “Molecular signature of anastasis for reversal of apoptosis,” F1000 Res, 6:43, 2017.
- G. Sun et al., “A molecular signature for anastasis, recovery from the brink of apoptotic cell death,” J Cell Biol, 216:3355–68, 2017.
- H.L. Tang et al., “In vivo CaspaseTracker biosensor system for detecting anastasis and non-apoptotic caspase activity,” Sci Rep, 5:9015, 2015.
- A.X. Ding et al., “CasExpress reveals widespread and diverse patterns of cell survival of caspase-3 activation during development in vivo,” eLife, 5:e10936, 2016.
- G. Sun, D.J. Montell, “Q&A: Cellular near death experiences—what is anastasis?” BMC Biol, 15:92, 2017.
- Y.N. Gong et al., “ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences,” Cell, 169:P286–300.e16, 2017.
- J.C. Hamann et al., “Entosis is induced by glucose starvation,” Cell Rep, 20:201–10, 2017.
Interested in reading more?