Stem cells could deliver tumor-busting drugs, repair damaged brains, and even mend a (literally) broken heart. But first, scientists must figure out how to get these jacks-of-all-trades where they’re needed and ensure that they survive long enough to do their work.
Achieving this objective requires being able to observe the cells after they’re set loose inside a living, breathing animal. Of course, scientists can make tissue slices and use histology to look for the cells, but that offers only a snapshot of one final time point.
“If you want to understand what happens to these stem cells, it’s important to track the fate of these cells without having to kill the animal,” says Joseph Wu, a cardiologist at Stanford University School of Medicine in Palo Alto, California. Stem cell transplants may settle down, proliferate, and differentiate as desired; they may form dangerous tumors; or they may simply falter and...
Researchers are harnessing advanced imaging to look inside their animals, or even inside patients, and track stem cells as they travel through tissues. Some techniques rely on inert tags the stem cells carry with them, and others use genetic markers that prove a cell is actually alive and making protein.
Each method has its advantages and disadvantages. “Depending on the research question, you may even want to combine different approaches,” says Frank Bengel, a nuclear medicine physician at Hannover Medical School in Germany.
Here, The Scientist profiles four methods used to track stem cells once they’re on their own inside a new body.
Optical Imaging: Bioluminescence
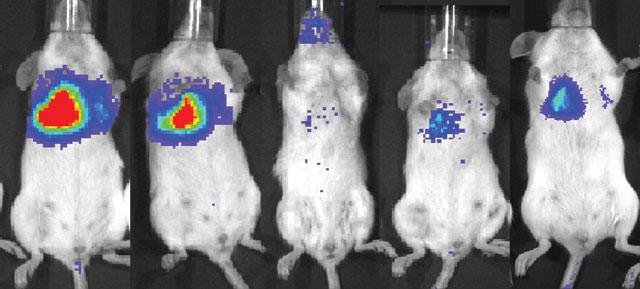 Luciferase-expressing mesenchymal stem cells, when injected intravenously into a mouse with lung metastases, are first trapped in the lung itself. The optical signal decreases over time but begins to increase after six days, suggesting that some stem cells are proliferating in or near the tumor cellsCOURTESY O FSHAWN CHEN
Luciferase-expressing mesenchymal stem cells, when injected intravenously into a mouse with lung metastases, are first trapped in the lung itself. The optical signal decreases over time but begins to increase after six days, suggesting that some stem cells are proliferating in or near the tumor cellsCOURTESY O FSHAWN CHEN User
Xiaoyuan (Shawn) Chen, chief and senior investigator, Laboratory of Molecular Imaging and Nanomedicine, National Institute of Biomedical Imaging and Bioengineering, Bethesda, Maryland.
Project
Ultimately, Chen hopes to develop personalized medical therapies, including those relying on stem cells. For now, he’s simply trying to understand the fate of transplanted stem cells, and how they home in on tumors—targets to which they might someday custom-deliver chemotherapy (Stem Cells, 27:1548-58, 2009).
Technique
Mesenchymal stem cells home in on tumors and divide there, promoting remodeling of the tissue—suggesting they might be good drug-delivery vehicles. Before transplanting mouse mesenchymal stem cells into breast tumor-bearing mice with lung metastases, the researchers engineered the cells to express luciferase. By injecting the mice with luciferin—the enzyme’s substrate—they caused the transplanted cells to light up. The researchers also included the gene for green fluorescent protein, so they could double-check their results by histology. Although most of the stem cells got stuck in the lung’s capillaries, a few not only survived, but also proliferated and differentiated near the tumor sites.
Resolution
3–5 mm
Pros
- “Bioluminescence imaging is the workhorse for small-animal imaging: it’s cheap, it’s easy to do, it’s high-throughput,” Wu says.
- Only living cells light up.
- The method is highly sensitive, detecting 1,000 or fewer cells. “The background is very low,” Chen adds.
Cons
- Bioluminescence can only penetrate through a few millimeters of tissue; it won’t work for large animals or people.
- The resulting image is flat, not 3-D.
- Some cells may turn off the transgene, producing false negatives.
Equipment
Bioluminescence imaging requires a light-tight box with fluorescence filters and camera. Caliper Life Sciences’ version costs $100,000–$400,000, depending on the features chosen.
Related Alternatives
- Fluorescent proteins
- Quantum dots
 MRI image showing a stroke involving the left temporal-occipital region (green).MEDICAL BODY SCANS / PHOTO RESEARCHERS, INC. COLORIZATION BY: MATTHEW BOLOGNA
MRI image showing a stroke involving the left temporal-occipital region (green).MEDICAL BODY SCANS / PHOTO RESEARCHERS, INC. COLORIZATION BY: MATTHEW BOLOGNAMagnetic Resonance Imaging
User
Marcel Daadi, neuroscientist and instructor in the neurosurgery department, Stanford University School of Medicine, Palo Alto, California.
Project
Daadi hopes to use stem cells to repair the neural networks of people who’ve suffered strokes or have Parkinson’s disease, thus improving their ability to move normally. An early step is to examine what happens to neural stem cells once grafted into the brains of rats that had had induced strokes (Mol Ther, 17:1282-91, 2009.)
Technique
Daadi’s team used both bioluminescence and MRI to follow the fate of human neural stem cells transplanted into the rats’ brains. To make the cells visible in an MRI, the researchers first filled them up with tiny magnetic beads. Many cell types readily take up these superparamagnetic iron oxide (SPIO) particles. Such grafts show up in the image as low-intensity areas. The researchers used bioluminescence imaging to confirm that the stem cells did indeed survive, and settled near the site of the stroke for as long as three months. The MRI results indicated that the more cells transplanted, the larger the graft that was formed.
Resolution
0.01–1.5 mm
Pros
- Anatomical information is built in. “It gives you great resolution of the brain…we see where the stroke is, where cells are in relation to the stroke,” Daadi says.
- Some SPIO particles are already FDA-approved, so the technique could be used in humans.
- It’s possible to use MRI in the operating room, so surgeons could confirm exactly where they injected the SPIO-labeled cells.
Cons
- Low sensitivity: Wu often asks his MRI-expert colleagues to blindly detect injected SPIO versus background. “A lot of times, they can’t,” he says.
- MRI gives no indication of cell viability—dead cells can hang around for weeks. Or, macrophages clean up the area, ingesting the SPIO and producing a false signal. “You could see the ghosts of cells, but not living cells,” says Eduardo Marbán, director of the Cedars-Sinai Heart Institute in Los Angeles, California.
- As the cells proliferate, the iron particles get diluted.
Equipment
For preclinical imaging, a 9.4 Tesla magnet is the “sweet spot,” says Rob Robinson, global business development manager for MRI at Agilent Technologies. Those machines, which range in price from $1.5 million to $3 million depending on size, are available at research institutions, hospitals, and at medical schools.
Clinical-grade magnetic nanoparticles are available from AMAG Pharmaceuticals, which markets Feraheme to treat anemia, and Miltenyi Biotec makes iron dextran beads for cell separation. BioPAL and Genovis provide experimental-grade SPIO for approximately $200–$500/2 mL, according to Joe Frank of the National Institutes of Biomedical Imaging and Bioengineering.
Related Alternatives
- Perfluorocarbon particles that show up when an MRI is tuned for fluorine
- MRI reporter genes that increase the iron content of cells (see below).
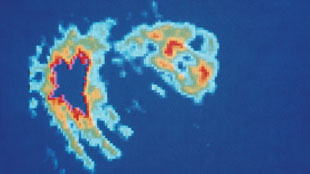 PET scan of the human heart showing the effect of a blood clot.SCIENCE SOURCE
PET scan of the human heart showing the effect of a blood clot.SCIENCE SOURCE User
Eduardo Marbán, director and Mark Siegel Family Foundation Chair, Cedars-Sinai Heart Institute, Los Angeles, California.
Project
Marbán, a cardiologist, aims to use stem cells to repair the muscle damage resulting from a heart attack. He hopes stem cells will provide the necessary signals to make the heart convert scar tissue into healthy muscle. Unfortunately, the heart is a tricky target for stem cells—once injected, the heart pumps them right back out again. Within an hour, only 10% of the graft is left in the heart, and only 1% of cells remain after three weeks, he laments. “If we could boost those values, then we could get more bang for the buck with stem-cell therapy,” Marbán says. In a recent study, he and his colleagues managed to improve retention rates by slowing or stopping the heart or dabbing a bit of fibrin glue in the injection site. (J Am Coll Cardiol, 54:1619-26, 2009).
Technique
The scientists transplanted cardiac-derived stem cells from healthy rats into the hearts of those that had undergone surgically-orchestrated heart attacks. To label the transplanted stem cells, they first incubated them with the radioactive tracer 18FDG, a form of glucose toting a radioactive fluorine, that was internalized by the cells. Using a PET scanner, they were able to follow the cells and show that after an hour, 20–75% of the transplanted cells remained in the heart if its beat was slowed by drugs or if glue was dabbed into the injection site.
Resolution
1–10 mm
Pros
- “It’s the only reliable quantitative method, other than destructive methods,” Marbán says, because the half-life of the tracer is known.
- PET is already available in clinics.
- Minimal background signal.
Cons
- PET is limited by the half-life of the tracer; signals from 18-fluorine, for example, last for six to eight hours because the isotope’s half-life is 110 minutes.
- PET does not indicate cell viability—the tracer could leak out of the cell or show up in macrophages that devoured the original stem cells.
- This method requires the use of radioactive substances, with the associated risks to cells, animals, and personnel.
Equipment
A PET scanner from Philips Healthcare runs between $1 million and $1.5 million, and the machines have become standard equipment in hospitals. Radioactive tracers are also required; a human-size dose of 18FDG costs $200-250 at Cedars-Sinai. To acquire the tracers, one needs to be within a few hours of a cyclotron that can generate the radioactive fluorine ion for coupling to the glucose; most major universities have one, Wu says.
Related Alternatives
- Single-photon emission computed tomography (SPECT)
- Combining PET with computed tomography helps scientists localize the radioactive signal within the animal’s anatomy.
Genetic Reporters for PET
User
Frank Bengel, chairman of the department of nuclear medicine, Hannover Medical School, Germany
Project
Both PET and MRI only reveal where the labels are. “The cell may die and you may still get the signal,” Bengel says. Plus, preloading the cells only works as long as the tracers last—hours or days for PET tracers. Bengel is developing reporter genes that allow living cells to produce their own PET signal at any time.
Technique
The technique relies on genes whose products force the cell to grab onto radioactive tracers. For example, the herpes simplex virus enzyme thymidine kinase (HSV-tk) phosphorylates a guanine homolog called FHBG. Unphosphorylated FHBG travels freely in and out of cells. But once phosphorylated—as only happens in cells having the HSV-tk gene—it’s negatively charged, and stuck on the inside. By engineering stem cells to express the transgene before transplantation, then treating the animal with radioactive FHBG, researchers can observe the transplants on a PET scan.
Resolution
1–10 mm
Pros
- Only live cells show up: “If you don’t see a signal, there are no cells,” Wu says.
- With promoters specific for different cell types, these reporters could also indicate whether the stem cells go on to differentiate.
- HSV-tk provides a built-in fail-safe: the viral kinase phosphorylates the antiviral drug ganciclovir, turning the drug cytotoxic. Should transplanted cells cause trouble—for example, start to form a tumor—doctors could treat the person with ganciclovir. Upon phosphorylation by HSV-tk, the drug would simply kill the genetically engineered cells.
Cons
- PET with reporters is not as sensitive as with directly radiolabeled cells; more cells are necessary to produce a detectable signal.
- The transgene might compromise the cell’s normal function, or the cell might silence the transgene.
- As a viral protein, HSV-tk may activate the host immune system.
Equipment
One needs a PET scanner plus cloning technology.
Related Alternatives
- Researchers are also working with mitochondrial thymidine kinase, the human sodium-iodide symporter (NIS), and the mammalian dopamine type 2 receptor as potential PET reporters.
- MRI reporters are also an option: overexpression of ferritin or of the transferrin receptor, both of which regulate cellular iron levels, makes the iron-rich cells show up.
Interested in reading more?





